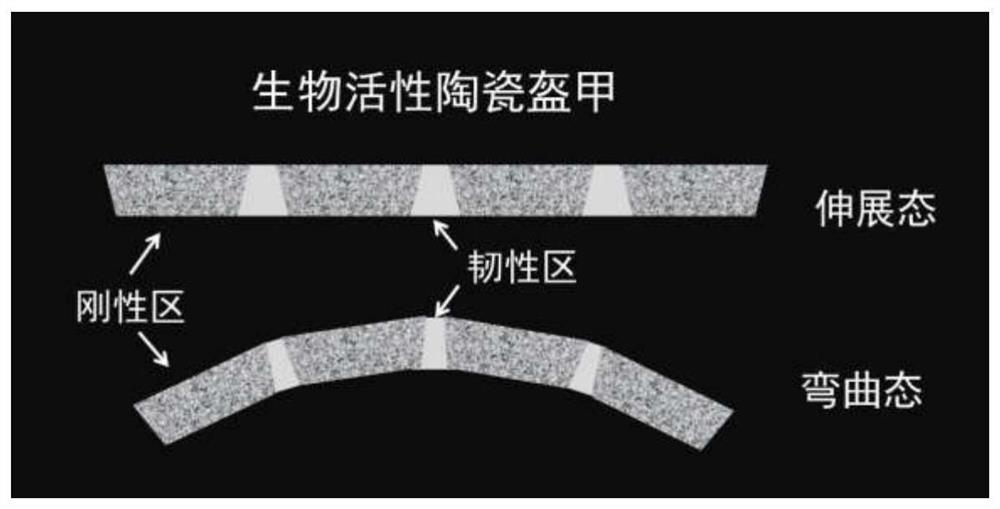
Mechanically adaptable bioactive ceramic armor and its preparation method
A bioactive ceramic and adaptive technology, applied in tissue regeneration, medical science, prosthesis, etc., can solve the problems of ignoring the adaptive adjustment of skull deformation and not being able to realize the bionic skull structure and functional process well
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) dissolving 1.00g of chitosan in an acetic acid solution with a volume fraction of 2%, and magnetic stirring for 30min under a water bath condition of 37° C., to obtain a chitosan acid solution;
[0036] (2) Add 3mL each of 2mol / L soluble calcium salt solution and 1.2mol / L soluble phosphate solution to the mixed solution of step (1) successively with a time interval of 30min; wherein the Ca / P molar ratio is n(Ca 2+ ):n(PO 4 3- )=1.67:1;
[0037] (3) At room temperature, quickly transfer the homogeneous mixture in step (2) to a hexagonal mold. After ultrasonically eliminating air bubbles, place it in a refrigerator at -20°C for 24 hours, and then transfer it to a freeze dryer. , freeze-dried at -109°C for 3 days;
[0038] (4) Submerge the freeze-dried molded sample in step (3) in an alkaline solution (1:1, v / v) with pH = 9, place it in a constant temperature oscillator at 37°C for 8 hours, and wash it with water until After neutralization, refreeze and then dry to...
Embodiment 2
[0044] (1) dissolving 1.00g of chitosan in an acetic acid solution with a volume fraction of 2%, and magnetic stirring for 30min under a water bath condition of 37° C., to obtain a chitosan acid solution;
[0045] (2) Each 6mL of 2mol / L soluble calcium salt solution and 1.2mol / L soluble phosphate solution is added in the mixed solution of step (1) successively, and the time interval is 30min; wherein the Ca / P molar ratio is n(Ca 2+ ):n(PO 4 3- )=1.67:1;
[0046] (3) At room temperature, quickly transfer the homogeneous mixture in step (2) to a hexagonal mold. After ultrasonically eliminating air bubbles, place it in a refrigerator at -20°C for 24 hours, and then transfer it to a freeze dryer. , freeze-dried at -109°C for 3 days;
[0047] (4) Submerge the freeze-dried molded sample in step (3) in an alkaline solution (1:1, v / v) with pH = 10, place it in a constant temperature oscillator at 37°C for 8 hours, and wash it with water until After neutralization, refreeze and the...
Embodiment 3
[0052] (1) dissolving 1.00g of chitosan in an acetic acid solution with a volume fraction of 2%, and magnetic stirring for 30min under a water bath condition of 37° C., to obtain a chitosan acid solution;
[0053] (2) Add 8 mL each of 2mol / L soluble calcium salt solution and 1.2mol / L soluble phosphate solution to the mixed solution of step (1) successively, with a time interval of 30min; wherein the Ca / P molar ratio is n(Ca 2+ ):n(PO 4 3- )=1.67:1;
[0054] (3) At room temperature, quickly transfer the homogeneous mixture in step (2) to a hexagonal mold. After ultrasonically eliminating air bubbles, place it in a refrigerator at -20°C for 24 hours, and then transfer it to a freeze dryer. , freeze-dried at -109°C for 3 days;
[0055] (4) Submerge the freeze-dried molded sample in step (3) in an alkaline solution (1:1, v / v) with pH = 10, place it in a constant temperature oscillator at 37°C for 12 hours, and wash it with water until After neutralization, refreeze and then dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com