Preparation method and application of alpha-GalCer nanoparticles
A nanoparticle and solution technology, applied in biochemical equipment and methods, microorganisms, blood/immune system cells, etc., can solve the problems of PET cytotoxicity and time-consuming, and achieve the effect of simple preparation process and short preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation method of α-GalCer nanoparticle
[0034] 1. Solution preparation:
[0035] Preparation of PEI solution: dissolve PEI with a molecular weight of 1800 in the diluent so that the final concentration of PEI is 1 mg / ml; preparation of α-GalCer solution: dissolve α-GalCer in the diluent so that the final concentration of α-GalCer is 1 mg / ml.
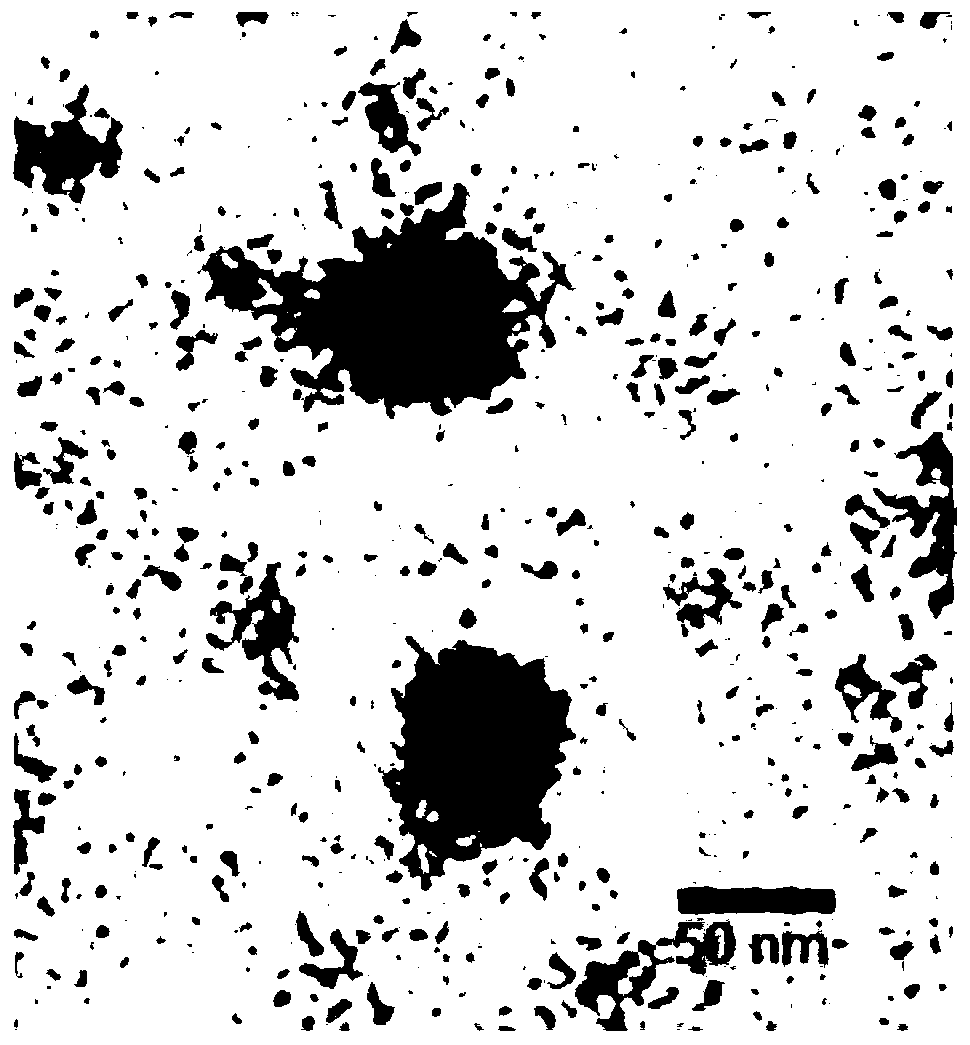
[0036] 2. Preparation of α-GalCer nanoparticles: α-GalCer and PEI can form nanoparticles through electrostatic interaction. Put the prepared PEI solution in a round-bottomed centrifuge tube, then slowly drop into the same volume of α-GalCer solution to mix, while gently vortexing the test tube, after mixing well, let stand at room temperature for 10-25 hours to form α-GalCer nano particle mixture. Take 2 μl of the solution and apply it on the copper grid, and after natural drying, observe the morphology of the nanoparticles with an electron microscope, as shown in figure 1 As shown, the results show tha...
Embodiment 2
[0039] Embodiment 2: cytotoxicity test
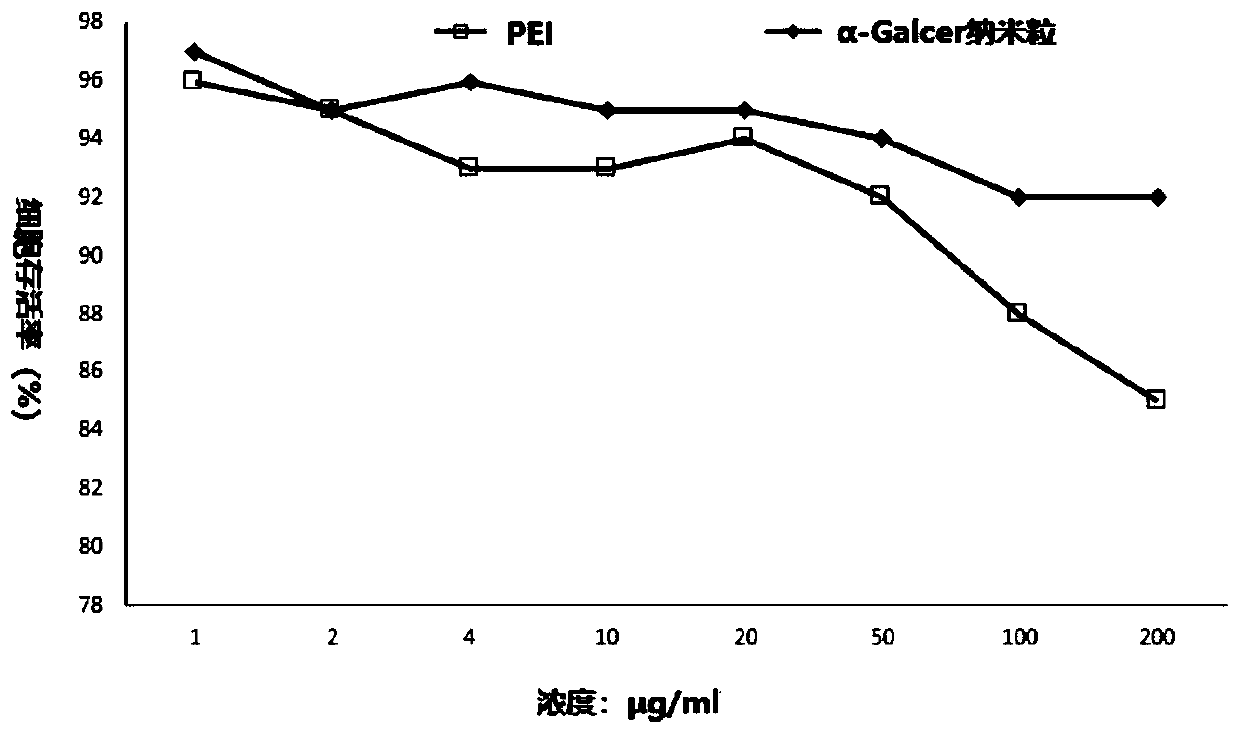
[0040] Peripheral blood mononuclear cells were seeded on a 96-well plate at a cell density of 2×10 4 / well, cultured. PEI or nanoparticles are formulated into different mass concentration gradients in the culture medium. Aspirate the original culture medium in each well, add different concentrations of PEI culture medium, 0.2ml per well, and incubate with the cells for 72 hours, and detect the growth of the cells by the cck8 method. The cell survival rate at different time points after the test was used to infer the toxicity of PEI and α-GalCer nanoparticles to mononuclear cells, and the feasibility of their application to cell induction has been judged. Experimental results such as figure 2 It was shown that α-GalCer nanoparticles reduced the cytotoxicity of PEI, and the cell survival rate was over 90%.
Embodiment 3
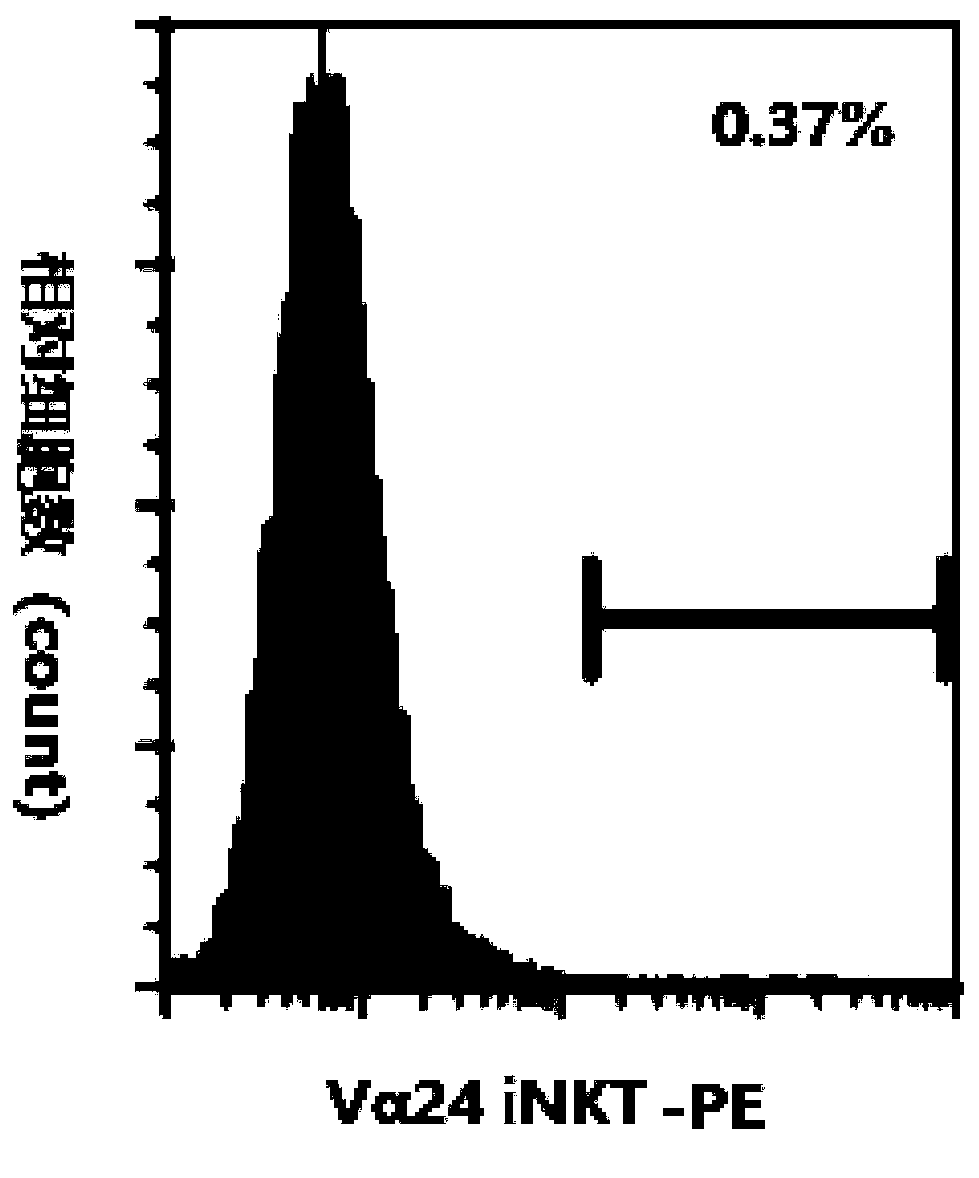
[0041] Example 3: α-GalCer nanoparticles stimulate iNKT proliferation experiment
[0042] 1. Day 0
[0043] i. Collect peripheral blood, pour the collected peripheral blood directly into a sterile centrifuge tube for 30-40ml, balance the centrifuge tube, centrifuge at 3500rpm for 10min. At the end of the centrifugation, slowly collect the upper plasma with a pipette and put it into another new sterile centrifuge tube, and the remaining part is used in the subsequent step b. The collected plasma was inactivated in a water bath at 56°C for 30 minutes, and the supernatant was collected by centrifugation for later use.
[0044] j. Dilute the rest with the same volume of normal saline (room temperature).
[0045] k. Add 15ml Ficoll (room temperature) to a 50ml conical tube. The top of the Ficoll is slowly covered with 25ml of diluted blood mixture. Centrifuge at 400 x g for 30 minutes without brake at room temperature.
[0046] l. Carefully remove the interface buffy coat and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com