Single cell whole genome sequencing method
A whole-genome sequencing and single-cell technology, applied in the field of molecular biology, can solve problems such as the application of single-molecule sequencing and single-cell whole-genome sequencing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1 Amplification method of the present invention Amplifies single cell genome and sequencing analysis
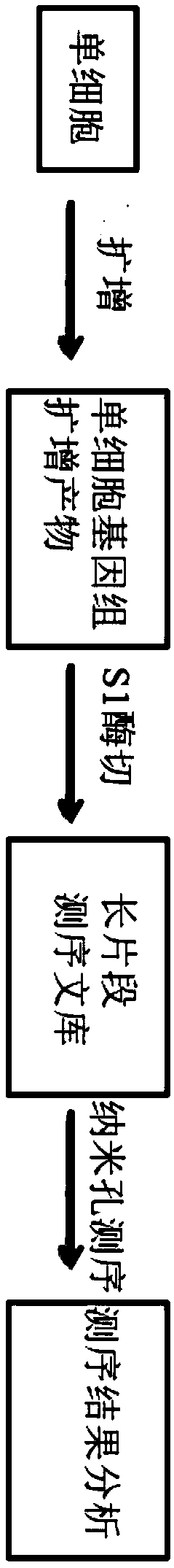
[0095] See the experimental procedure figure 1 , the specific experimental steps and results are as follows:
[0096] 1. Obtain single cells
[0097] (1) K562 (human chronic myeloid leukemia cell line) cells were revived and cultured to 10 6 / mL.
[0098] (2) Use a capillary to pick a single cell into a PCR tube.
[0099] 2. Extract single-cell genome and amplify it
[0100] An amplification system was prepared using a single cell expansion kit (REPLI-g single cell kit (Qiagen, Cat. No. 150343)).
[0101] (1) Take 33 μL Reconstituted Buffer DLB and add 3 μL dithiothreitol (DTT(1M)) to configure buffer D2.
[0102] (2) Add 3 μL buffer D2 to 4 μL single cell suspension, mix gently, and centrifuge.
[0103] (3) Incubate at 65°C for 10 minutes.
[0104] (4) Add 3 μL of stop solution, mix gently, and centrifuge to obtain a single cell lysate.
[0105] (5...
Embodiment 2
[0119] Example 2 The microdroplet-based amplification method of the present invention amplifies single-cell genome and sequence analysis
[0120] See the experimental procedure Figure 6 , the specific experimental steps and results are as follows:
[0121] 1. Obtain single cells
[0122] (1) K562 (human chronic myeloid leukemia cell line) cells were revived and cultured to 10 6 / mL.
[0123] (2) Use a capillary to pick a single cell into a PCR tube.
[0124] 2. Extract single-cell genome and amplify it
[0125] An amplification system was prepared using a single-cell genome amplification kit (REPLI-gsinglecellkit, Qiagen, Cat. No. 150343).
[0126] (1) Take 33 μL Reconstituted Buffer DLB and add 3 μL dithiothreitol (DTT, 1M) to configure buffer D2.
[0127] (2) Add 3 μL buffer D2 to 4 μL single cell suspension, mix gently, and centrifuge.
[0128] (3) Incubate at 65°C for 10 minutes.
[0129] (4) Add 3 μL of stop solution, mix gently, and centrifuge to obtain a single...
Embodiment 3
[0148] Embodiment 3 comparative experiment
[0149] 1. Bulk genomic DNA extraction.
[0150] 1.1 K562 (human chronic myeloid leukemia cell line) cells were revived and cultured to 10 6 / mL.
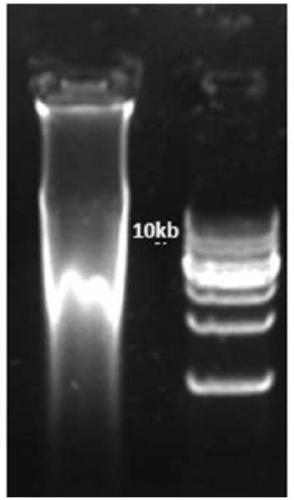
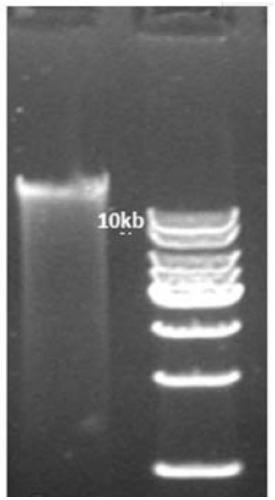
[0151] 1.2 Take 2×10 6 cells, and the genomic DNA was extracted by the spin column method. The total DNA mass was 20 μg detected by absorbance (nanodrop). 1% agarose gel electrophoresis running gel results see Figure 10 .
[0152] 2. Send the bulk genomic DNA to Wuhan Future Group Company for sequencing.
[0153] 3. The results of bulk genome DNA sequencing are considered as the original sequence before amplification and serve as the genome reference sequence. Comparing the original sequence with the sequencing results of the single-cell amplification method and the microdroplet-based amplification method can evaluate the probability of base mutation and chromosome structure variation during the amplification process of the two single-cell genome amplification methods , so as to ju...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com