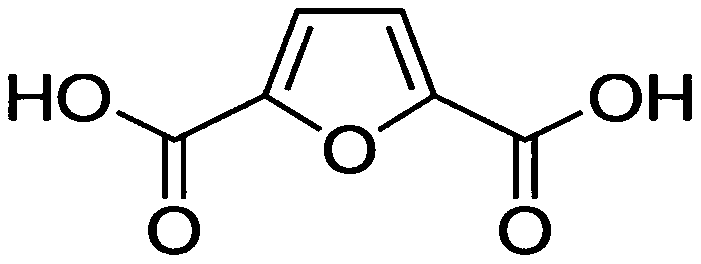
Device and method for preparing 2, 5-furandicarboxylic acid from hexose diacid (hexose diacid salt) by coupling dehydration cyclization reaction and azeotropic distillation dehydration
A technology of furandicarboxylic acid and cyclization reaction, applied in chemical instruments and methods, azeotropic distillation, separation methods and other directions, can solve problems such as reduction and impact on yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
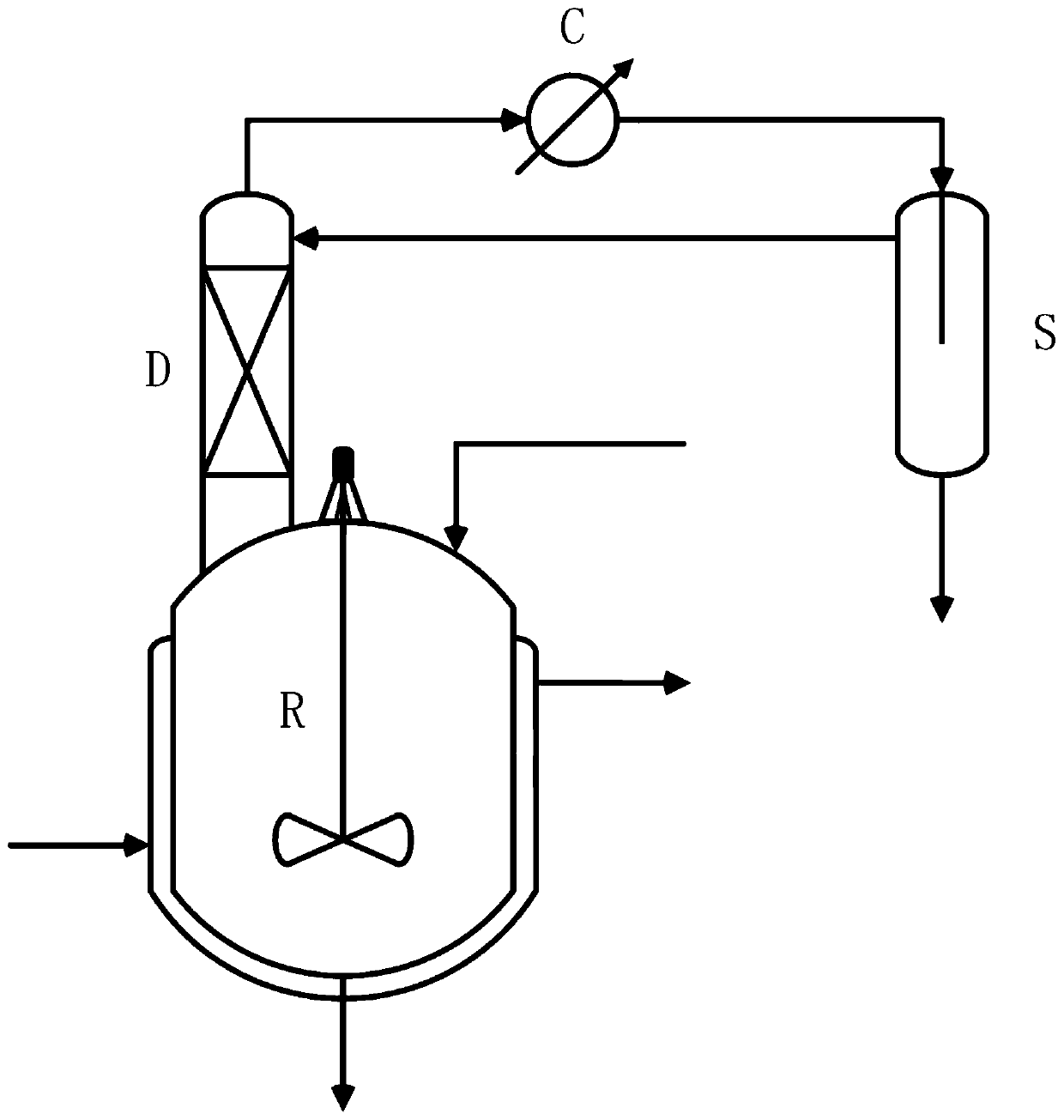
[0026] The first aspect of the present invention provides a device for preparing 2,5-furandicarboxylic acid from hexaric acid (salt) coupled with dehydration cyclization reaction and azeotropic distillation to remove water. The device includes the following components: dehydration cyclization Reactor R, rectification tower D, condenser C, phase separator S, wherein the dehydration and ring closure reactor R is connected to rectification tower D, and the top of rectification tower D is connected to condenser C and phase separator S, and phase separator S is connected with rectification column D.
[0027] The second aspect of the present invention provides a method for preparing 2,5-furandicarboxylic acid from hexaric acid (salt) coupled with dehydration ring closure reaction and azeotropic distillation to remove water, the method comprising the following steps:
[0028] 1) Open the reactor to stir and heat the reactor jacket to heat the steam, and add reaction solvent, hexaric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com