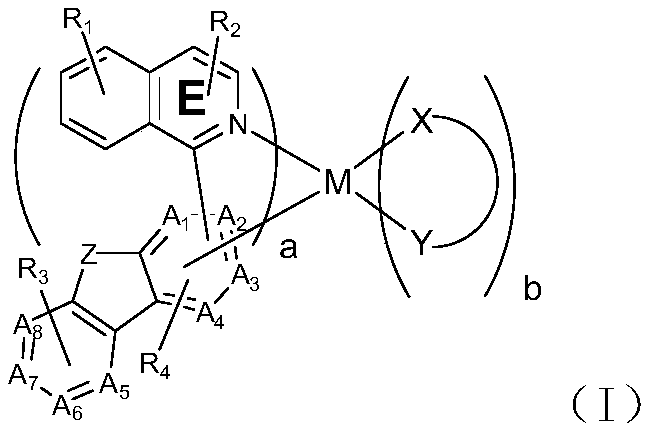
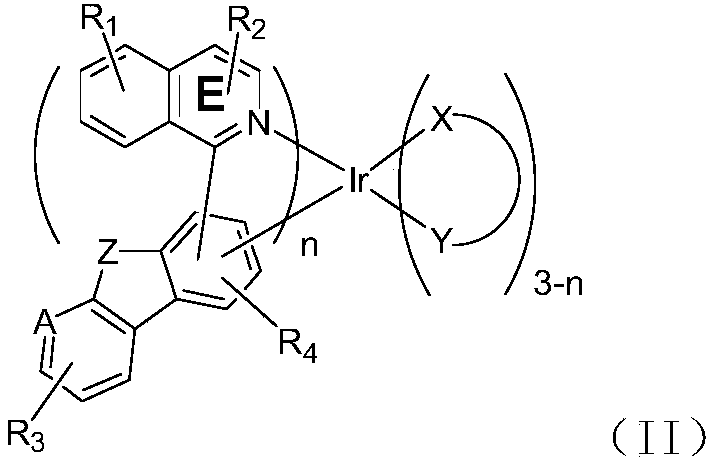
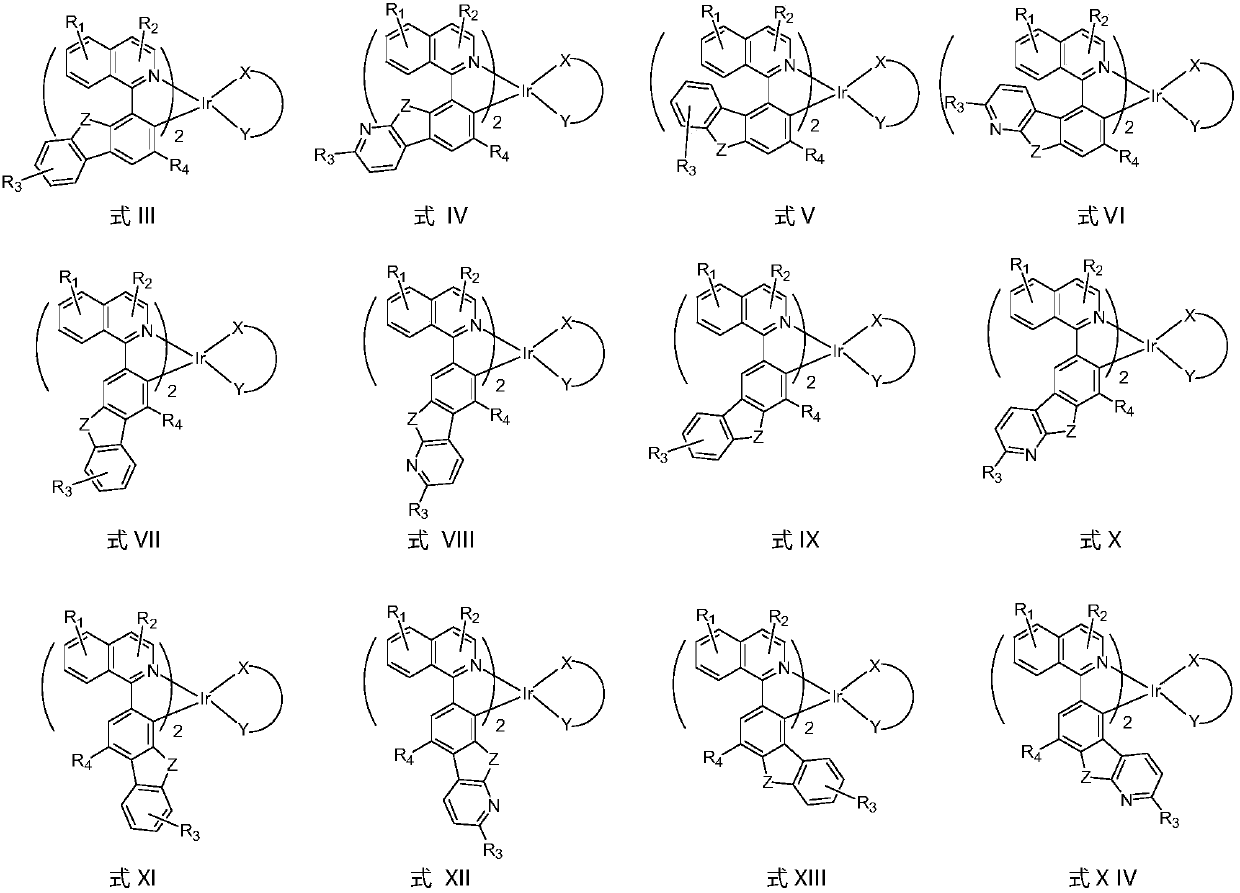
Organometallic compound and application thereof
An organometallic and compound technology, applied in indium organic compounds, platinum group organic compounds, organic chemistry, etc., can solve problems such as high sublimation temperature, poor color saturation, and unsatisfactory device performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of shared intermediates:
[0041]
[0042] Synthesis of compound 2:
[0043] Compound 1 (27.2g, 0.12mol, 1.2eq), 1-chloroisoquinoline (16.3g, 0.1mol, 1.0eq) and Cs 2 CO 3 (97.5g, 0.3mol, 3.0eq) and Pd(dppf)Cl 2 (3.655g, 0.05mol, 0.05eq) into a flask, add anhydrous toluene 1L, N 2 Under protection, stir and reflux at 110°C for 16 hours. Cool to room temperature, concentrate to remove the organic solvent, add 1L dichloromethane and 1L water to the residue, stir and separate the layers, and the organic phase is successively washed with H 2 O (500ml*3) and saturated aqueous sodium chloride solution (500ml*3) were washed, dried over sodium sulfate, filtered, and the filtrate was concentrated to remove the organic solvent. An off-white solid was obtained, which was purified by slurrying with EtOH to obtain compound 2 (24.33 g, yield: 78.7%). Mass spectrum: 310.12 (M+H), 1HNMR: 8.42 (d, 1H), 7.92 (dd, 1H), 7.87 (dd, 1H), 7.71 (dd, 1H), 7.50-7.57 (m, 3H), 7.3...
Embodiment 2
[0053] Synthesis of shared intermediates:
[0054]
[0055] Synthesis of compound 5:
[0056] Compound 4 (28.92g, 0.12mol, 1.2eq), 1-chloro-6 isopropylisoquinoline (20.5g, 0.1mol, 1.0eq) and Cs 2 CO 3 (97.5g, 0.3mol, 3.0eq) and Pd(dppf)Cl 2 (3.655g, 0.05mol, 0.05eq) into a flask, add anhydrous toluene 1L, N 2 Under protection, stir and reflux at 110°C for 16 hours. Cool to room temperature, concentrate to remove the organic solvent, add 1L dichloromethane and 1L water to the residue, stir and separate the layers, and the organic phase is successively washed with H 2 O (500ml*3) and saturated aqueous sodium chloride solution (500ml*3) were washed, dried over sodium sulfate, filtered, and the filtrate was concentrated to remove the organic solvent. An off-white solid was obtained, which was purified by column chromatography with ethyl acetate / petroleum ether to obtain compound 5 (28.12 g, yield: 76.8%). Mass spectrum: 367.18 (M+H), 1HNMR: 8.4 (d, 1H), 7.81 (dd, 1H), 7.7...
Embodiment 3
[0087] Synthesis of CPD 79:
[0088]
[0089] Synthesis of compound 9:
[0090] Compound 8 (29.1g, 0.12mol, 1.2eq), 1-chloroisoquinoline (16.3g, 0.1mol, 1.0eq) and Cs 2 CO 3 (97.5g, 0.3mol, 3.0eq) and Pd(dppf)Cl 2 (3.655g, 0.05mol, 0.05eq) was put into a flask, and 1L of anhydrous toluene was added, under the protection of N2, stirred and refluxed at 110°C for 16 hours. Cool to room temperature, concentrate to remove the organic solvent, add 1L dichloromethane and 1L water to the residue, stir and separate the layers, and the organic phase is successively washed with H 2 O (500ml*3) and saturated aqueous sodium chloride solution (500ml*3) were washed, dried over sodium sulfate, filtered, and the filtrate was concentrated to remove the organic solvent. An off-white solid was obtained, and the solid was separated by column chromatography to obtain compound 9 (23.41 g, yield: 72.1%). Mass spectrum: 326.1(M+H), 1HNMR: 8.51(d,1H), 7.71-7.82(m,5H), 7.50-7.60(m,3H), 7.30-7.4(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com