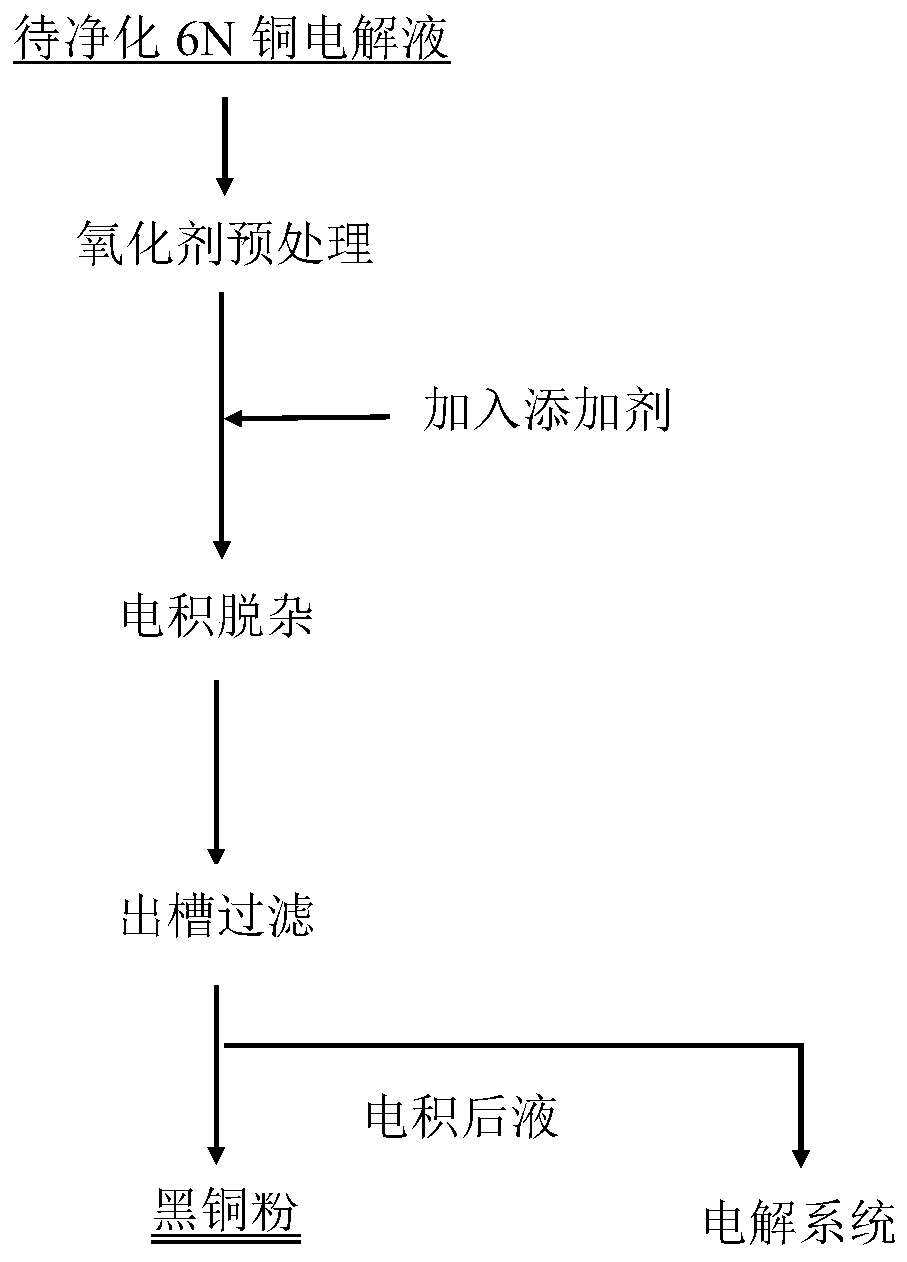
Electrodeposition impurity removing process for 6N copper electrolyte
A copper electrolyte and electrolyte technology, applied in the field of metallurgy, can solve the problems of poor stability of impurity removal by adsorption method, high consumption of raw materials, long process flow, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
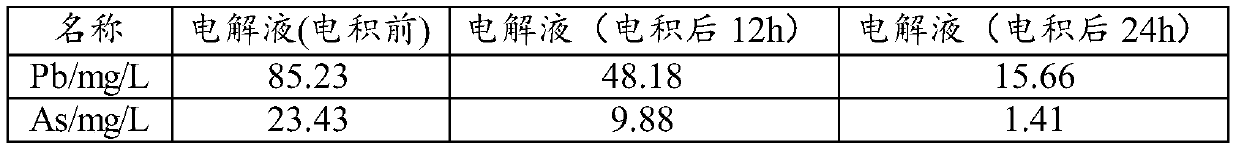
[0052] Take Cu 2+ 40L of 6N copper electrolyte with a concentration of 100g / L, add 650ml of hydrogen peroxide in the lower tank, pre-oxidize at 20°C for 10 hours, and control the current density at 250A / m 2 , the electrodeposition temperature is 30°C, and the electrodeposition time is 24h.
[0053] The purified electrolyte composition and black powder composition are shown in Table 1 and Table 2
[0054] Electrolyte composition after purification in the embodiment of the present invention 1 in table 1
[0055]
[0056]
[0057] The composition of black powder in the embodiment of the present invention 1 of table 2
[0058]
Embodiment 2
[0060] Take Cu 2+ 40L of copper nitrate electrolyte with a concentration of 150g / L, 1L of hydrogen peroxide was added to the lower tank, oxidation pretreatment was carried out at 30°C for 7 hours, and the current density was controlled at 550A / m 2 , the electrodeposition temperature is 30°C, and the electrodeposition time is 24h.
[0061] The purified electrolyte composition and black powder composition are shown in Table 1 and Table 2
[0062] Electrolyte composition after purification in the embodiment of the present invention 1 in table 1
[0063] name Electrolyte (before electrowinning) Electrolyte (12h after electrowinning) Electrolyte (24h after electrowinning) Pb / mg / L 108.91 45.34 19.20 As / mg / L 36.53 12.17 1.68 Sb / mg / L 28.71 9.33 0.97 Bi / mg / L 24.31 7.83 1.36
[0064] The composition of black powder in the embodiment of the present invention 1 of table 2
[0065]
Embodiment 3
[0067] Take Cu 2+ Concentrations of 50g / L and 150g / L copper nitrate electrolyte to be purified are 40L each, add 1L of hydrogen peroxide to the lower tank, pre-oxidize at 30°C for 7 hours, and control the current density to 550A / m 2 , the electrodeposition temperature is 30°C, and the electrodeposition time is 24h.
[0068] Electrolyte composition after purification in the embodiment of the present invention 3 in table 3
[0069]
[0070]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com