A kind of norvancomycin hydrochloride preparation for injection and preparation method thereof
A technology for norvancomycin and injection, which is applied in the preparation methods of peptides, chemical instruments and methods, and pharmaceutical formulations, etc., can solve the problems of poor stability, many impurities, and low purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
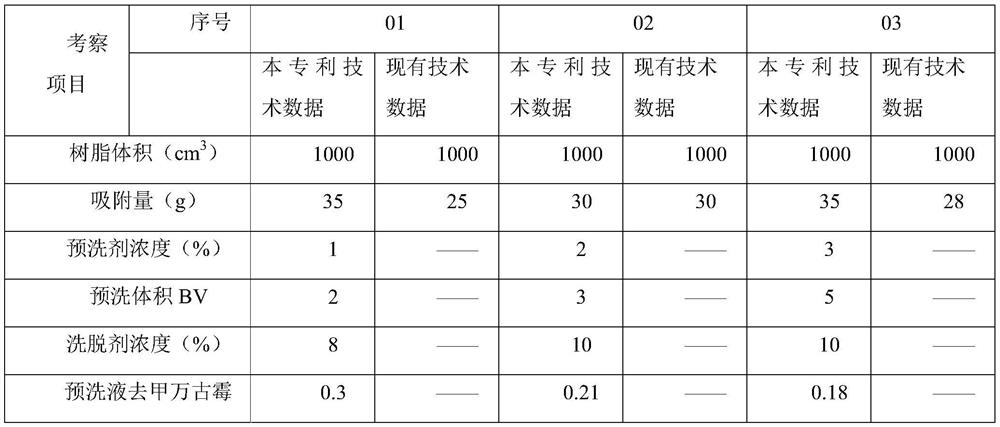
[0031] A. Chromatography: The new polymer filler is UniPMM40, with a volume of 200L, and a total of 6.131 billion units of norvancomycin feed solution is passed into it at a flow rate of 2BV / h, and the top water is 2min;
[0032] B. Pre-washing: prepare 1% methanol solution, adjust the pH to 7.2 with phosphoric acid, and pre-wash 2BV at a flow rate of 2BV / h;
[0033] C. Elution: prepare 10% methanol solution, adjust the pH to 7.0 with phosphoric acid, pass it into the chromatographic column at a flow rate of 2BV / h, and collect the eluate;
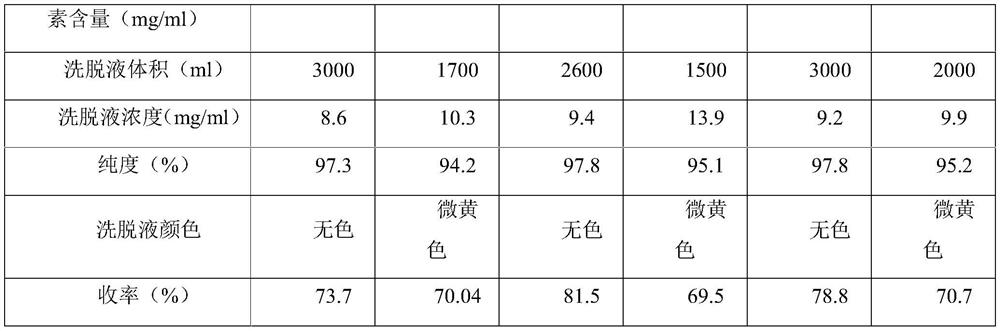
[0034] D. Membrane system concentration: After the eluate is concentrated by the membrane system, the residual solvent is washed away with purified water to obtain a vancomycin hydrochloride elution concentrate with a purity of 97.9%, colorless, and a yield of 76.6%.
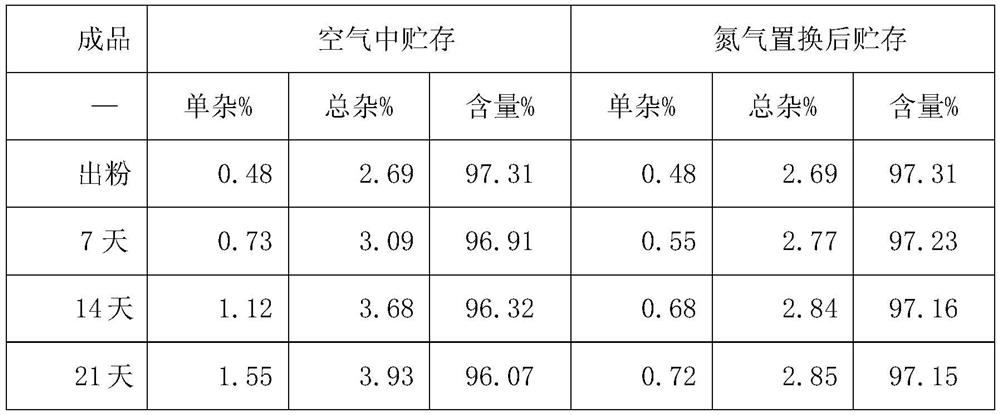
[0035] E. The finished solution is freeze-dried, taken out of the box, and sub-packaged. After nitrogen replacement, the oxygen concentration is 1.24%.
Embodiment 2
[0037] A. Chromatography: The new polymer filler is UniPMM40, with a volume of 200L, and a total of 6.056 billion units of norvancomycin feed solution is passed into it at a flow rate of 3BV / h, and the top water is 2min;
[0038] B. Pre-washing: Prepare 1.5% ethanol solution, adjust the pH to 7.2 with phosphoric acid, and pre-wash 4BV at a flow rate of 2BV / h;
[0039] C. Elution: prepare 11% ethanol solution, adjust the pH to 7.0 with phosphoric acid, pass it into the chromatographic column with a flow rate of 3BV / h, and collect the eluent;
[0040] D. Membrane system concentration: After the eluate was concentrated by the membrane system, the residual solvent was washed away with purified water to obtain the vancomycin hydrochloride elution concentrate, with a purity of 97.5%, colorless, and a yield of 74.8%.
[0041] E. The finished solution is freeze-dried, taken out of the box, and sub-packaged. After nitrogen replacement, the oxygen concentration is 1.39%.
Embodiment 3
[0043] A. Chromatography: The new polymer filler is UniPSN40, with a volume of 200L, and a total of 4.621 billion units of norvancomycin feed solution is introduced at a flow rate of 2BV / h, and the top water is 2min;
[0044] B. Pre-washing: prepare 1% methanol solution, adjust the pH to 7.1 with phosphoric acid, and pre-wash 2BV at a flow rate of 2BV / h;
[0045] C. Elution: Prepare 8% methanol solution, adjust the pH to 7.0 with phosphoric acid, pass it into the chromatography column at a flow rate of 2BV / h, and collect the eluate;
[0046] D. Membrane system concentration: After the eluate is concentrated by the membrane system, the residual solvent is washed away with purified water to obtain the vancomycin hydrochloride elution concentrate, with a purity of 97.1%, colorless, and a yield of 71.2%.
[0047] E. The finished solution is freeze-dried, taken out of the box, and subpackaged, and the oxygen concentration is 1.15% after nitrogen replacement.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com