Red phosphorescent host compound and organic light emitting device using same
A red phosphorescence and compound technology, applied in the field of soluble phosphorescence host compounds and OLED devices, can solve problems such as lack of materials, and achieve the effects of easy availability of raw materials, reduction of vibration energy loss, and high-efficiency luminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of compound 1
[0035] Synthesis of Intermediate Sub-1
[0036]
[0037] In the 500mL reaction flask, add intermediate phenylboronic acid (7.49g, 61.4mmol), 2,4-dibromothiazole (17.68g, 61.4mmol), tetrakis (triphenylphosphine) palladium (5mol%), K2CO3 (17.0g , 122.8mmol), 1,4-dioxane (200mL) and water (50mL). The temperature of the reaction system was raised to 80° C., and reacted for 12 hours under the protection of nitrogen. After the reaction was completed, the reaction solution was cooled to room temperature, and extracted with o-dichlorobenzene and water. The organic layer was dried over anhydrous magnesium sulfate, concentrated, and recrystallized to obtain the crude product through a silica gel column to obtain intermediate Sub-1 (13.13 g, yield 75%) LC-MS: M / Z 283.99 (M+H)+
[0038] Synthesis of compound 1
[0039]
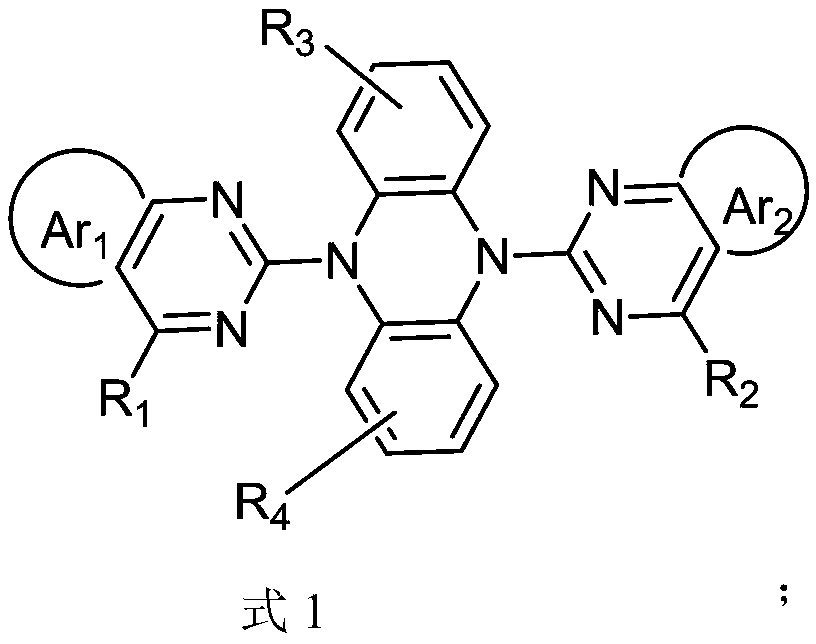
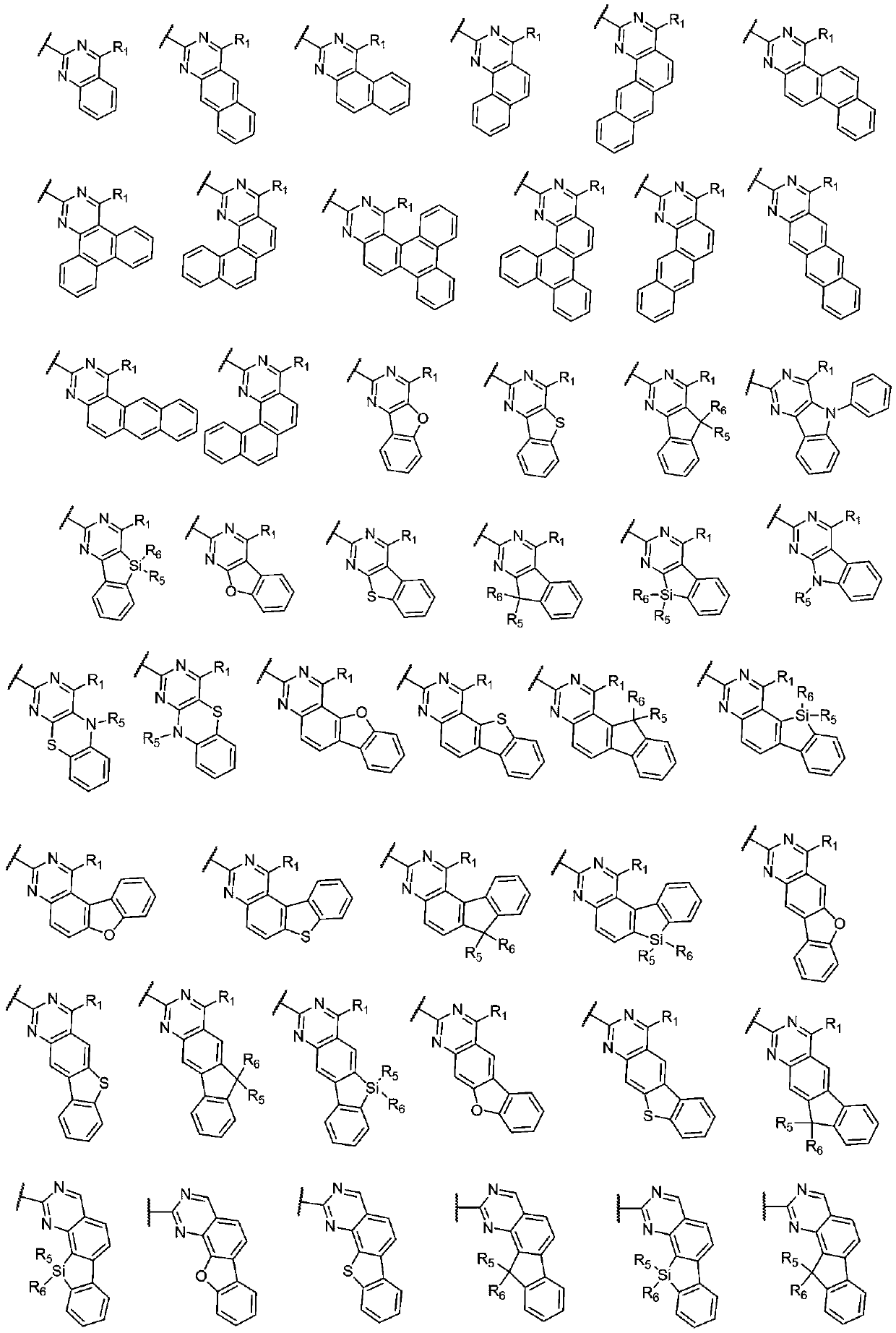
[0040] In a 250mL three-necked flask, add intermediate 5,10-dihydrophenazine (1.82g, 10.0mmol), intermediate ...
Embodiment 2
[0041]Embodiment 2: the synthesis of compound 14
[0042] Synthesis of Intermediate Sub-2
[0043]
[0044] Add intermediate 2,4-dibromobenzo[4,5]thieno[3,2-d]pyrimidine (3.85g, 11.2mmol) and intermediate carbazole (3.0g, 12.3mmol) in a 250mL three-necked flask , tris(dibenzylideneacetone)dipalladium (4 mol%), tri-tert-butylphosphine (8 mol%), potassium tert-butoxide (3.8 g, 33.6 mmol) and o-xylene (80 mL). The temperature of the reaction system was raised to 120° C. and reacted for 12 hours under the protection of nitrogen. After the reaction was completed, the reaction solution was cooled to room temperature, and extracted with o-dichlorobenzene and water. The organic layer was dried over anhydrous magnesium sulfate, concentrated, and the crude product obtained by recrystallization was passed through a silica gel column to obtain Sub-2 (3.86 g, yield 80%). LC-MS: M / Z 430.32 (M+H)+.
[0045] Synthesis of Compound 14
[0046]
[0047] Compound 14 was synthesized wit...
Embodiment 3
[0048] Embodiment 3: the synthesis of compound 22
[0049]
[0050] Compound 22 was synthesized with reference to the method of Example 2, and other steps were referred to the synthesis of Example 2 to obtain Compound 22 (6.55 g, yield 55%). LC-MS: M / Z 1062.30 (M+H)+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com