Method for obtaining RANKL recombinant protein with biological activity and application of RANKL recombinant protein with biological activity
A biologically active and recombinant protein technology, applied in chemical instruments and methods, animal/human proteins, medical preparations containing active ingredients, etc., can solve the problems of inhibition of osteoclast bone resorption and poor treatment effect of patients , to achieve good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Investigate the induced expression of RANKL recombinant protein 1. The induced expression of RANKL recombinant protein
[0044] Resuscitate BL21 competent cells containing pET-32a-mRANKL recombinant plasmid, inoculate in 0.5ml LB medium containing Amp resistance; 37°C, 150rpm shaking culture overnight, take 10ul bacterial liquid evenly plated in LB solid state culture Plates were incubated overnight at 37°C. Select monoclonal colonies for plate picking, inoculate in 5ml LB medium containing Amp resistance at 37°C and shake at 150rpm / min, culture overnight, inoculate 500ml LB medium containing Amp resistance at a ratio of 1:100, 37 ℃, shake the bacteria at 250rpm until the OD is between 0.4-0.6, add IPTG for induction for 5h, and extract the protein of the bacterial fluid.
[0045] 2. Extraction of RANKL recombinant protein
[0046] Centrifuge at 3000rpm for 5min to collect the bacteria, discard the supernatant, add PBS to resuspend, centrifuge at 3000rpm fo...
Embodiment 2
[0049] Example 2, Purification and Identification of RANKL Recombinant Protein
[0050] 1. Purification of RANKL recombinant protein
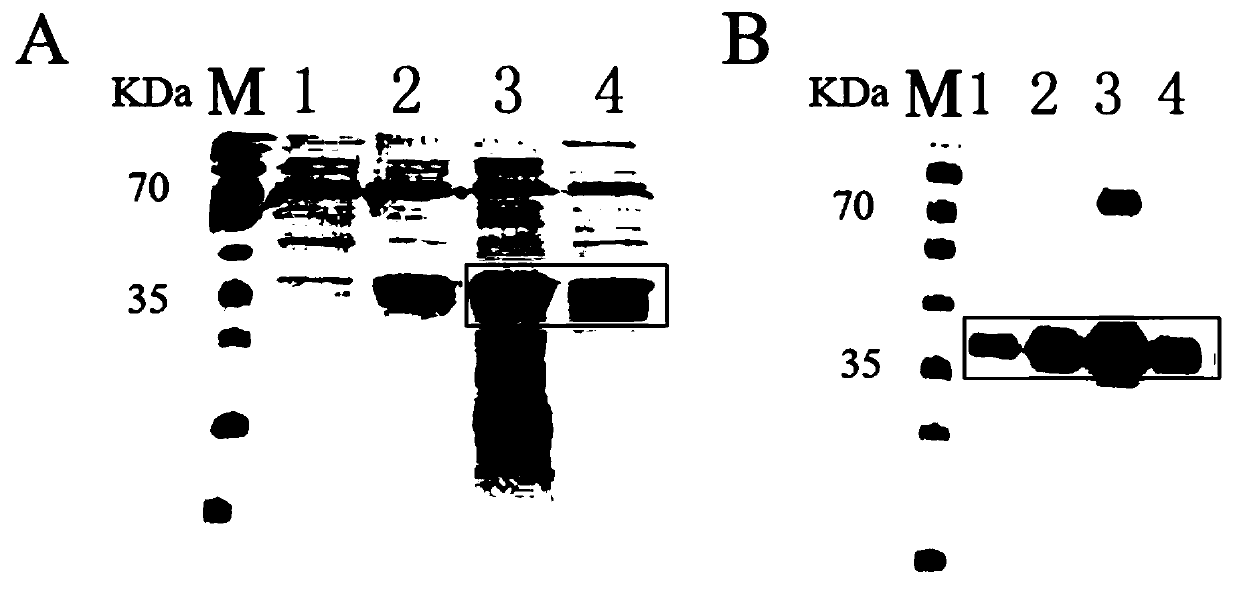
[0051] The extracted RANKL recombinant protein was purified through the His tag, that is, a peptide segment containing 6 histidines was added to the RANKL recombinant protein during design. Recombinant protein was purified.
[0052] 2. Identification of purified RANKL recombinant protein by SDS-PAGE
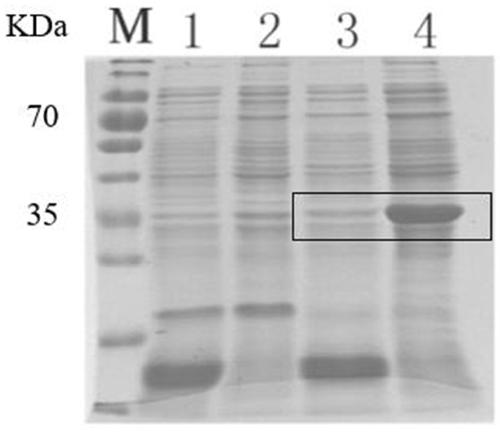
[0053] Electrophoresis was performed on 10% SDS-PAGE gel, and samples were loaded in the order of purified control supernatant protein sample, purified control precipitated protein sample, purified RANKL recombinant protein supernatant sample and purified RANKL recombinant protein precipitated sample, each Lane 40ug protein. After 120V for 90min, carry out Coomassie Brilliant Blue G-250 staining, the result is as follows figure 2 As shown in A, by Coomassie Brilliant Blue staining, it was found that the RANKL recombinant protein was mainly ex...
Embodiment 3
[0056] Example 3, Purification and Identification of RANKL Recombinant Protein
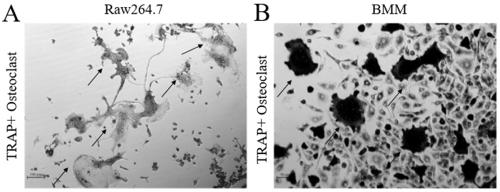
[0057] 1. RANKL recombinant protein induces osteoclast precursor Raw264.7 cells to differentiate into osteoclasts
[0058] Raw264.7 cells were seeded in 96-well cell culture plates, 10,000 cells / well, cultured in α-MEM medium with 10% fetal bovine serum (FBS), added 50ng / ml RANKL recombinant protein, 37°C 5% Osteoclasts were induced for 5-6 days under CO2 saturated humidity. Stained by tartrate-resistant acid phosphatase (TRAP) staining kit, photographed, and the photographed results were as follows: image 3 As shown in A, the cell staining is wine red. The irregular shaped cells containing multiple nuclei are osteoclasts, which contain filopodia and lamellipodia structures. Mature osteoclasts also contain folded border structures. The black cut-off head refers to the osteoclasts induced and differentiated by Raw264.7 cells.
[0059] 2. RANKL recombinant protein induces bone marrow macrophages...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com