Method for determining genotoxic impurities in doxofylline bulk drugs
A determination method and genotoxicity technology are applied in the field of determination of genotoxic impurities in doxofylline raw materials, and achieve the effects of reducing the loss of fixative solution, purifying test background and ensuring accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] 1) Sample preparation:
[0094] Preparation of mixed standard series solutions: Accurately weigh an appropriate amount of each reference substance, and use N,N-dimethylformamide to prepare vinyl acetate concentration of 3.846mg / ml, 2-bromo-1,1-dioxyethane concentration It is 0.3167mg / ml single reference substance stock solution, accurately measure 1ml of single reference substance stock solution into the same 100ml volumetric flask, add N,N-dimethylformamide to dilute and dilute to the mark to make a mixed control Sample stock solution, and then accurately draw and mix appropriate amount of reference stock solution, dilute to vinyl acetate concentration of 0.769μg / ml, 1.23μg / ml, 1.54μg / ml, 1.92μg / ml, 2.31μg / ml, 2- Bromo-1,1-dioxyethane concentrations are 0.0633μg / ml, 0.101μg / ml, 0.127μg / ml, 0.158μg / ml, 0.190μg / ml mixed standard series solutions;
[0095] Preparation of the test sample: Accurately weigh 0.3 g of the crude product of doxofylline (this sample is a simulat...
Embodiment 2-7
[0109] Except that the preparation of the test sample adopts the following method, the same measurement conditions and steps as in Example 1 are adopted.
[0110] Preparation of the test product: Accurately weigh 0.3 g of the crude product and finished product of doxofylline (provided by Shaanxi Bosen Bio-Pharmaceutical Co., Ltd.) into a 10 ml volumetric flask, add appropriate amount of N,N-dimethylformamide, shake and mix well , seal and sonicate for 1min until the sample is completely dissolved, add N,N-dimethylformamide to the mark, shake well, pass through a 0.22μm filter membrane, and the filtrate is used as the test sample.
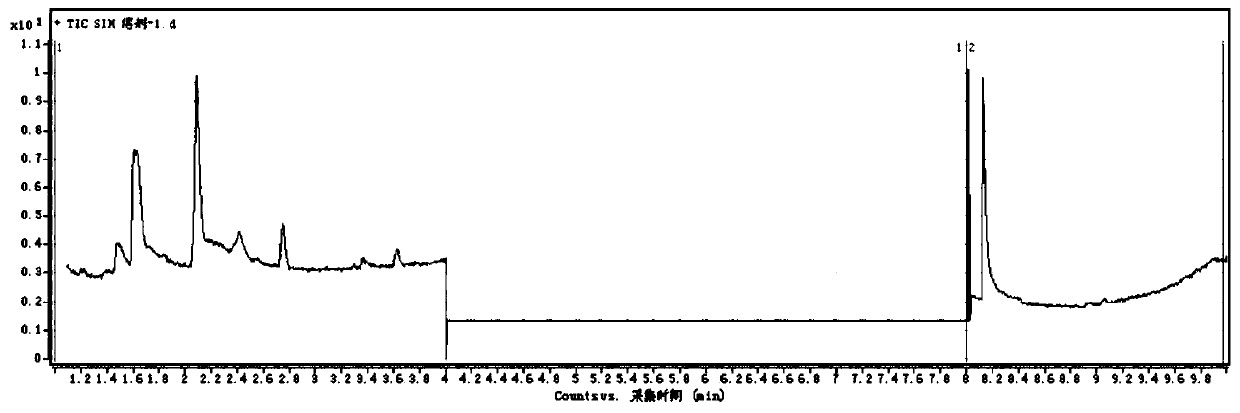
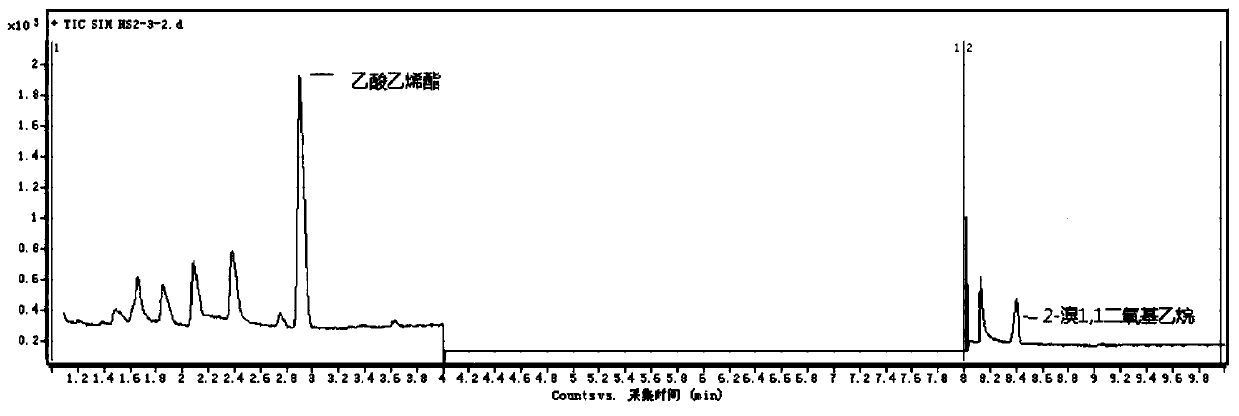
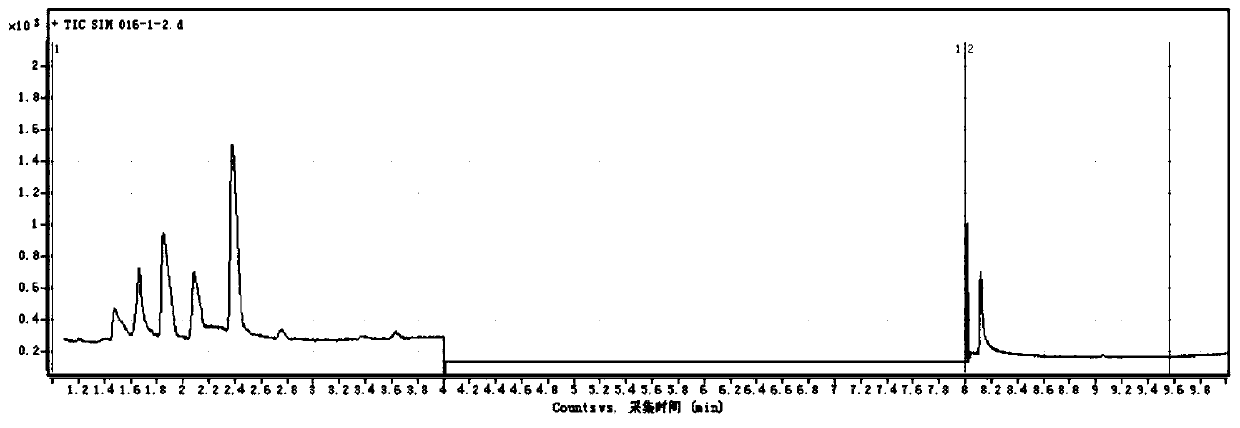
[0111] The measured gas chromatogram is as image 3 shown. image 3 It is the gas chromatography-mass spectrometry analysis spectrum of the sample.
[0112] The content determination results of the samples are shown in Table 2-7 below.
[0113] Table 2 Sample content determination results
[0114]
[0115] Table 3 Sample content determination...
Embodiment 3
[0127] Embodiment 3 methodological investigation
[0128] The specificity, linearity, precision, repeatability, accuracy, stability, detection limit and quantification limit of the method for the determination of two genotoxic impurities in doxofylline bulk drug were investigated. The specific inspection methods are:
[0129] 1. Linear inspection
[0130] Accurately weigh the appropriate amount of each reference substance, and use N,N-dimethylformamide to prepare vinyl acetate concentrations of 0.769 μg / ml, 1.23 μg / ml, 1.54 μg / ml, 1.92 μg / ml, and 2.31 μg / ml , 2-bromo-1,1-dioxyethane concentrations are respectively 0.0633μg / ml, 0.101μg / ml, 0.127μg / ml, 0.158μg / ml, 0.190μg / ml mixed standard series solutions (according to the increasing concentration The sequence is referred to as std1 solution, std2 solution, std3 solution, std4 solution and std5 solution). Inject the mixed standard series solution into the gas chromatograph-mass spectrometer, measure the corresponding peak area...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com