A kind of favipiravir granule preparation and preparation method thereof
A technology of Lavir granules and granule preparations, which is applied in the field of medicine, can solve the problems that tablets are difficult to swallow, and achieve the effects of good suspension effect, improved taste and prolonged residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0057] The used prescription and prescription consumption of embodiment 1-4 are as shown in table 1 below:
[0058] Table 1
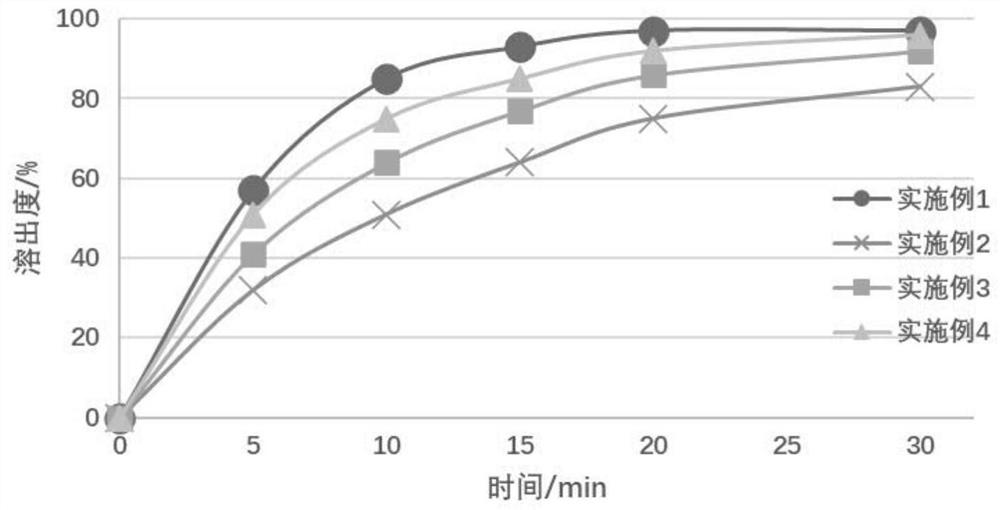
[0059] Component classification name Example 1 Example 2 Example 3 Example 4 parts by weight parts by weight parts by weight parts by weight Main drug Favipiravir 200 200 200 200 filler dextrin 0 200 500 100 suspending agent Tragacanth Gum 10 50 100 150 sweetener sucrose 2000 1300 800 0 sweetener aspartame 0 20 40 80 pH regulator citric acid 1 50 70 120 pH regulator Sodium citrate 10 75 30 20 Glidant Micropowder silica gel 50 50 50 20 Batch / gram 300 300 300 300 Main drug particle size / micron 9.4 38 29 12 Granule formulation pH 5.8 4.9 3.5 2.3 Sedimentation volume ratio 0.91 0.94 0.95 0.96 Taste score 3.1 points 3.7 points 4.3 points 3.5 points
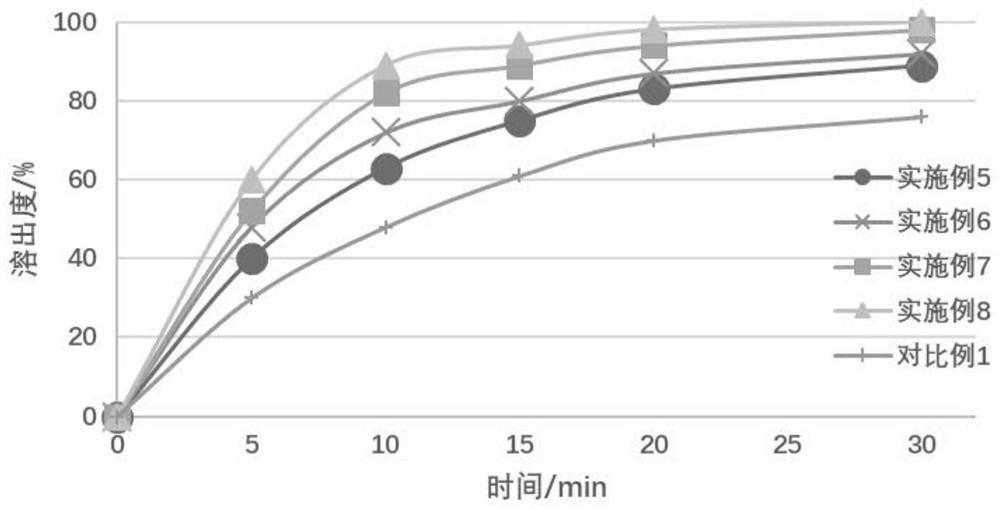
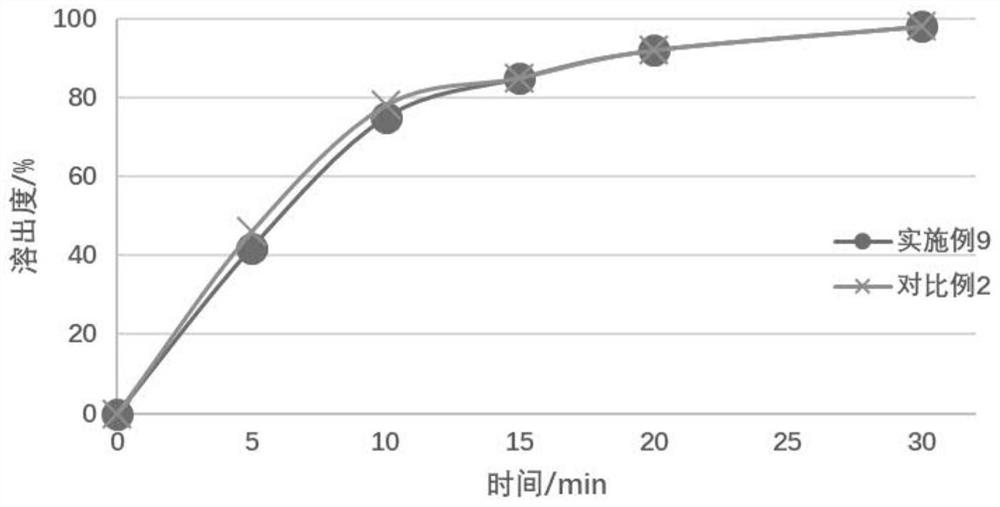
[0060] From the above resu...
Embodiment 1
[0061] Embodiment 1 preparation process steps:
[0062] 1) Favipiravir is subjected to airflow pulverization, the materials are collected, the particle size distribution is measured and controlled, and the D90 is obtained as a bulk drug of 9.4 microns;
[0063] 2) Pass tragacanth gum, sucrose, and micropowdered silica gel through a 60-mesh sieve respectively, and set aside;
[0064] 3) Mix the materials of step 1) and step 2) together in a mixer for 20 minutes to obtain the favipiravir granule formulation.
Embodiment 2~4
[0065] Embodiment 2~4 preparation process steps:
[0066] 1) Favipiravir is subjected to airflow pulverization, the material is collected, and the particle size distribution D90 is measured and controlled to be less than 40 microns;
[0067] 2) Pass the components other than the bulk drug through a 40-mesh sieve respectively, and set aside;
[0068] 3) Mix the other materials except micropowder silica gel and aspartame in step 1) and step 2) in a high-shear granulator, and then add the solution prepared by aspartame and purified water and continue stirring. Get the soft material with suitable wetting degree;
[0069] 4) the soft material of step 3) is carried out wet granulation by using a swing granulator with 40 meshes;
[0070] 5) The material obtained in step 4) is dried, and the obtained dried granules are dry granulated using a 40-mesh screen with an aperture;
[0071] 6) Mix the glidant and the granules obtained in step 5 in a mixer for 10 minutes to obtain the favip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com