Cobalt-cerium-manganese composite oxide catalyst for hydrogen production by autothermal reforming of acetic acid
A composite oxide, autothermal reforming technology, used in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, hydrogen/syngas production, etc., can solve the problem of catalyst deactivation, easy oxidation , not resistant to sintering and other problems, to achieve the effect of promoting migration, eliminating carbon deposits and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Weigh 1.547g of Co(NO 3 ) 2 ·6H 2 O, 6.081g of Ce(NO 3 ) 3 ·6H 2 O, 5.398g of 50% Mn(NO 3 ) 2 solution, add 35mL of deionized water to prepare solution #1; weigh 6.182g of NaOH and 1.024g of anhydrous Na 2 CO 3 , add 165mL of deionized water to prepare solution #2; under the condition of 78°C and pH of the solution at 10.5±0.5, add solution #1 and solution #2 dropwise into the beaker and keep stirring for co-precipitation reaction, and continue stirring Aging for 18 hours; after the aging, the mixture was suction-filtered, the obtained precipitate was filtered out and washed repeatedly with deionized water until the pH reached 7.0, and the obtained precipitate was dried in a drying oven at 105°C for 12 hours to obtain a catalyst precursor. The resulting precursor was calcined at 600°C at a heating rate of 10°C / min in a tubular resistance furnace for 4 hours to obtain a CDUT-CCM-1 catalyst; the chemical composition of the catalyst was (CoO 1.5 ) a (CeO 2 ) b ...
Embodiment 1
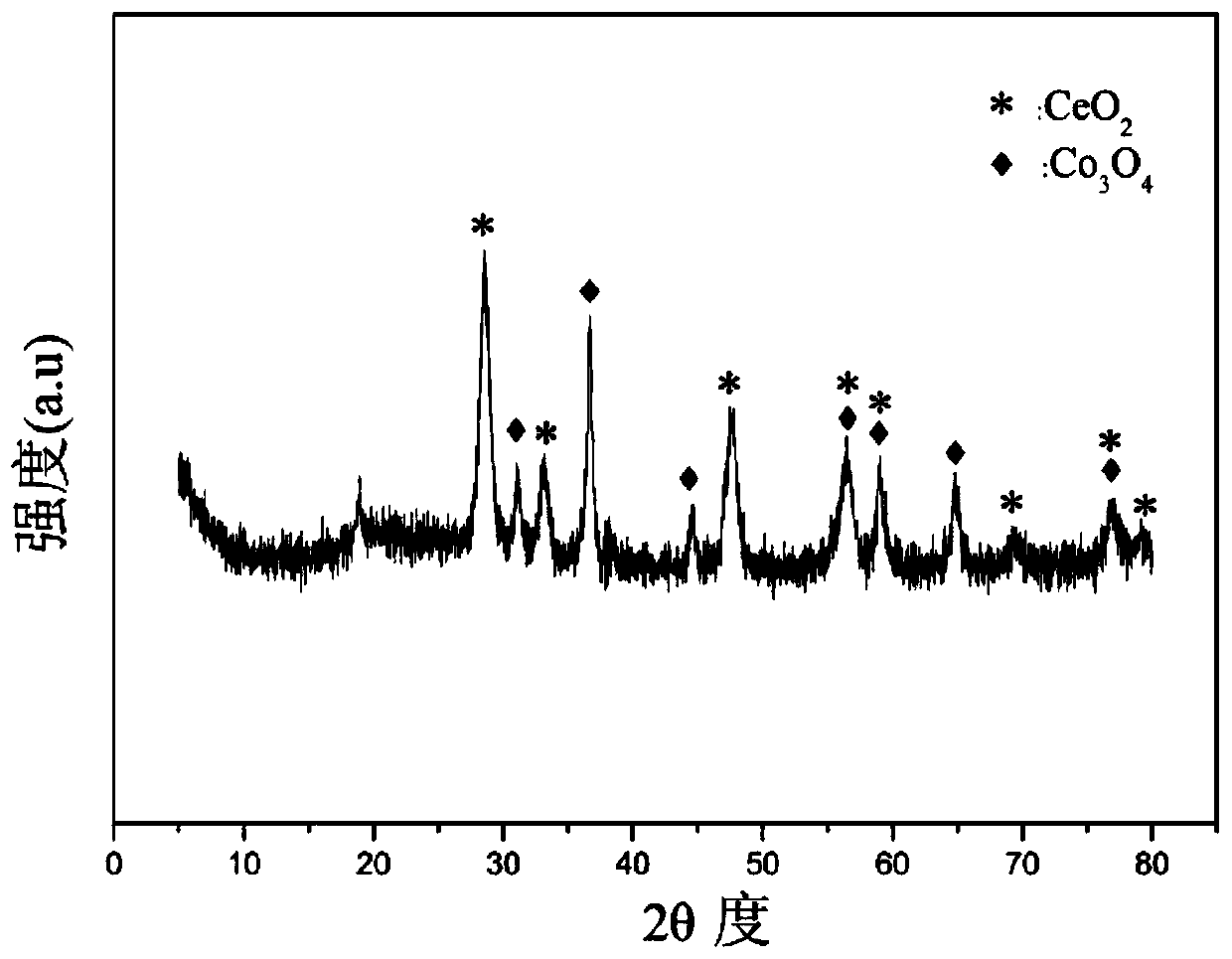
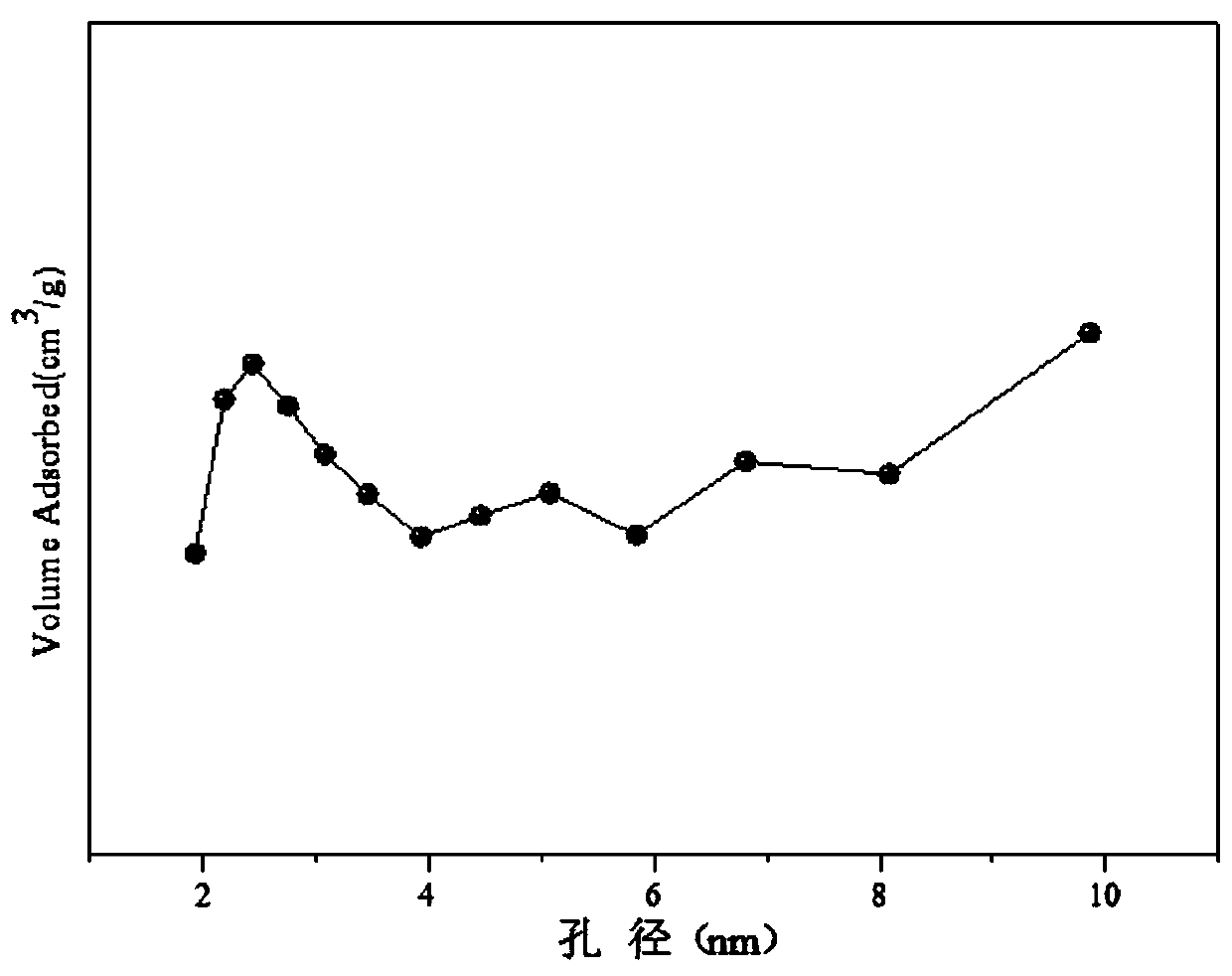
[0032] Weigh 1.558g of Co(NO 3 ) 2 ·6H 2 O, 8.013g of Ce(NO 3 ) 3 ·6H 2 O, 1.915g of 50% Mn (NO 3 ) 2 solution, add 30mL of deionized water to prepare solution #1; weigh 7.618g of NaOH and 1.262g of anhydrous Na 2 CO 3 , adding 203mL of deionized water to make solution #2; the subsequent steps are the same as in Reference Example 1, and the obtained precipitate was calcined at 600°C for 4 hours to obtain the CDUT-CCM-2 catalyst, whose typical structure is shown in the XRD spectrum in the attached figure 1 As shown, its main component is Co-containing 3 o 4 and Mn species embedded in CeO 2 Structure Formed Composite Oxide Solid Solution Ce-Mn-O x , formed Co / Ce-Mn-O after reduction x Composite oxide solid solution catalyst; the typical pore size distribution of its mesoporous structure is shown in the attached figure 2 shown; the chemical composition of the catalyst is (CoO 1.5 ) a (CeO 2 ) b (MnO) c , where a is 0.18, b is 0.64, and c is 0.18; the weight per...
Embodiment 2
[0035] Weigh 3.123g of Co(NO 3 ) 2 ·6H 2 O, 17.150g of Ce(NO 3 ) 3 ·6H 2 O, 1.805g of 50% Mn (NO 3 ) 2 solution, add 56mL of deionized water to prepare solution #1; weigh 16.070g of NaOH and 2.662g of anhydrous Na 2 CO 3 , add 427mL of deionized water to prepare solution #2; the subsequent steps are the same as in Reference Example 1. After the obtained precipitate is calcined at 600°C for 4 hours, the composite oxide solid solution CDUT-CCM-3 catalyst is obtained. Its typical structure is shown in XRD spectrum The picture is attached figure 1 As shown, the typical pore size distribution of its mesoporous structure is shown in the attached figure 2 shown; the molar composition of the catalyst is (CoO 1.5 ) a (CeO 2 ) b (MnO) c , wherein a is 0.19, b is 0.72, and c is 0.09, and the weight percentage in terms of oxides is composed of: cobalt oxide is 10%, cerium oxide is 85%, and manganese oxide is 5%.
[0036] The activity of the obtained CDUT-CCM-3 catalyst was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com