Method for generating functional noradrenaline neurons through reprogramming
A technology of norepinephrine, neurons, applied in the field of biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1, construction of lentiviral vector and lentiviral packaging
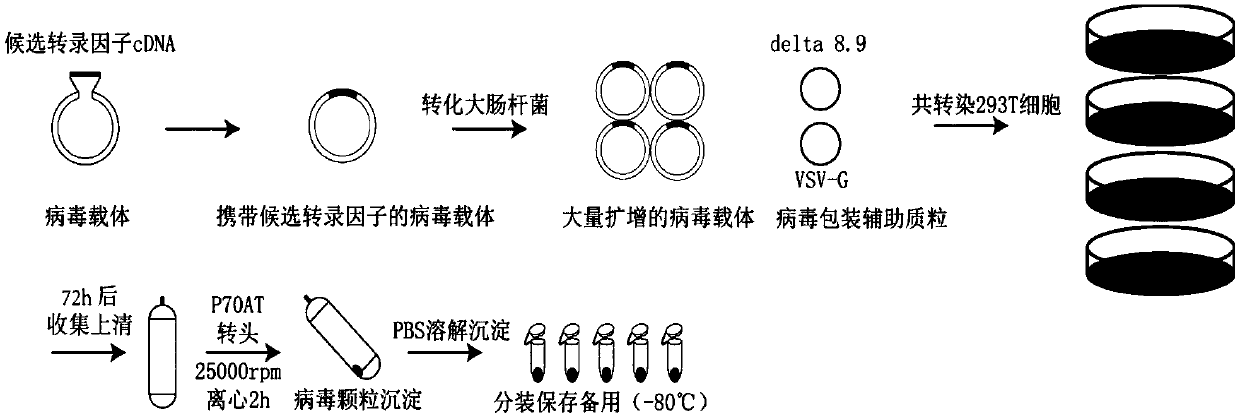
[0077] The schematic diagram of the construction of lentiviral vector and the operation process of lentiviral packaging is as follows: figure 1 shown. The cDNA of the candidate transcription factor was cloned into the lentiviral vector FUGW (vector capable of expressing green fluorescent protein), and sequenced to confirm that the cloned cDNA was correct. The obtained viral vector carrying the transcription factor was transformed into Escherichia coli for massive amplification and co-transfected with the other two viral packaging helper plasmids pCMV-dR8.91 (Delta 8.9) and pCMV-VSV-G into 293T cells for viral packaging. The lentiviral supernatant was concentrated by ultracentrifugation, and the resulting virus pellet was dissolved and then divided into devices at -80°C for later use.
Embodiment 2
[0078] Embodiment 2, preparation and purity identification of primary cells
[0079] The experimental mice were buried in crushed ice for about 5 minutes to make them dizzy, and the experimental mice were quickly soaked in 75% alcohol to disinfect the experimental mice. The head of the mouse was cut off and immediately transferred to a 10 cm Petri dish containing ice-cold dissecting fluid, and the skin of the head was cut off. Place the separated head under a dissecting microscope to remove the skull, dissect out the whole brain, and transfer it to a new 10cm petri dish containing dissecting fluid. The cerebellum and cerebrum were removed under the dissecting microscope, and the midbrain was retained, and the dorsal midbrain tissue was separated by cutting along the medullary foramen to both sides. After carefully removing the meninges with pointed tweezers, place the dorsal midbrain tectum tissue in a new 3.5cm petri dish, wash it three times with dissection solution, and fi...
Embodiment 3
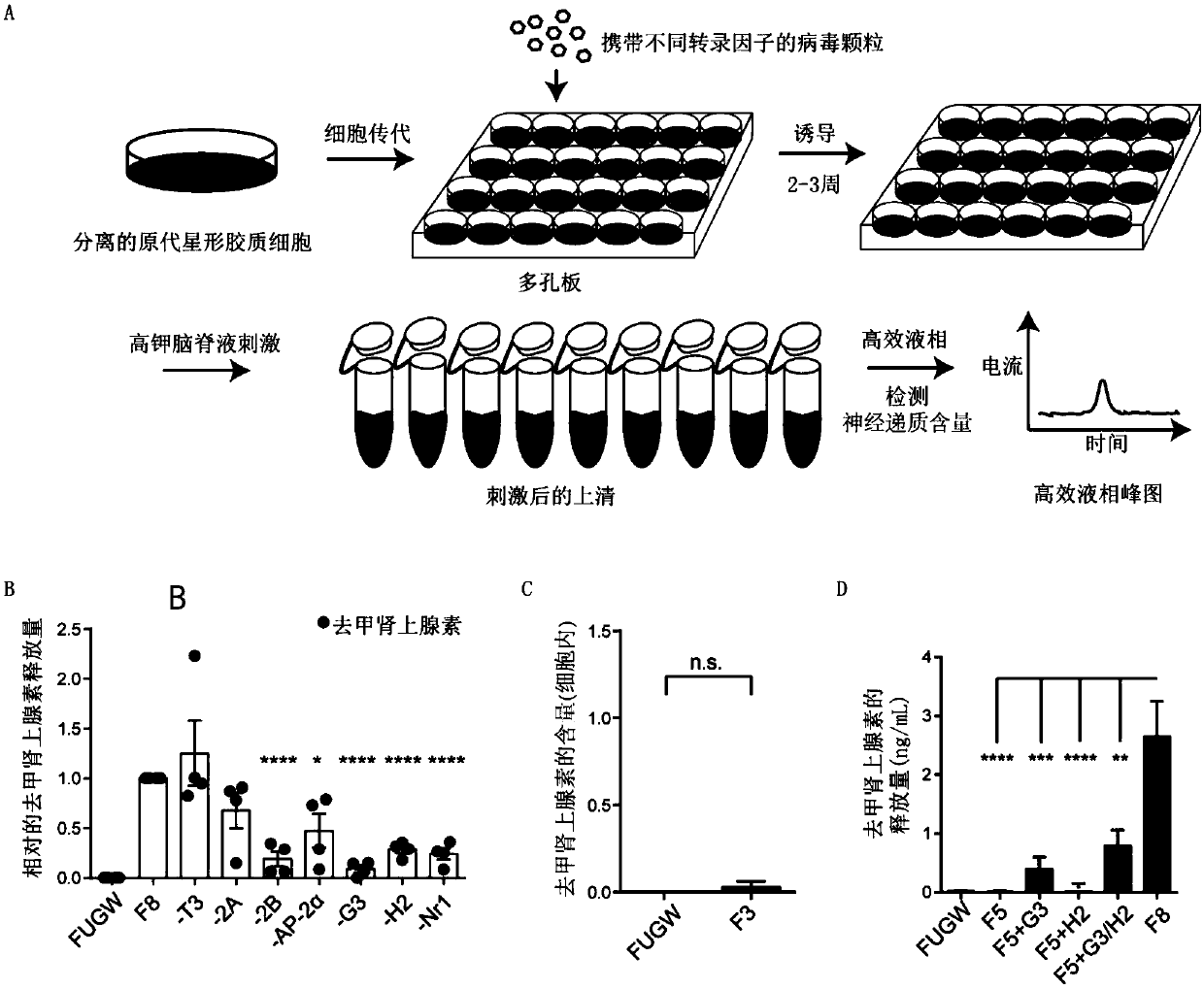
[0081] Example 3. Virus infection of primary cells for induction and factor screening
[0082] The inventors considered many factors at the initial stage of the experiment, and pre-screened them.
[0083] The day before induction, poly-D-lysine (10 μg / ml) was used to coat cell culture glass discs overnight at 37°C, and the glass discs were placed in 24-well plates. On the day of induction, the coated glass slices were washed three times with ultrapure water, dried in an ultra-clean bench, and then coated with laminin (10 μg / ml), at 37°C for 2-4h. Then the cultured glial cells or fibroblasts were planted on glass slides in 24-well plates at a density of 0.75×10 per well. 5 cell. The virus infection can be carried out 2 hours after the cells are plated, and the virus dosage is calculated in advance (the infection multiple of each virus is about 10 times the cell volume, which can be calculated according to the cell density and virus titer). After 12-16 hours of infection, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com