Lacosamide medicine oral preparation and preparation method thereof
A technology for lacosamide and oral preparations, applied in the field of medicine, can solve the problems of loose and brittle particles, unstable tableting, poor fluidity and the like, and achieve the effects of stable product quality, little impact and good safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
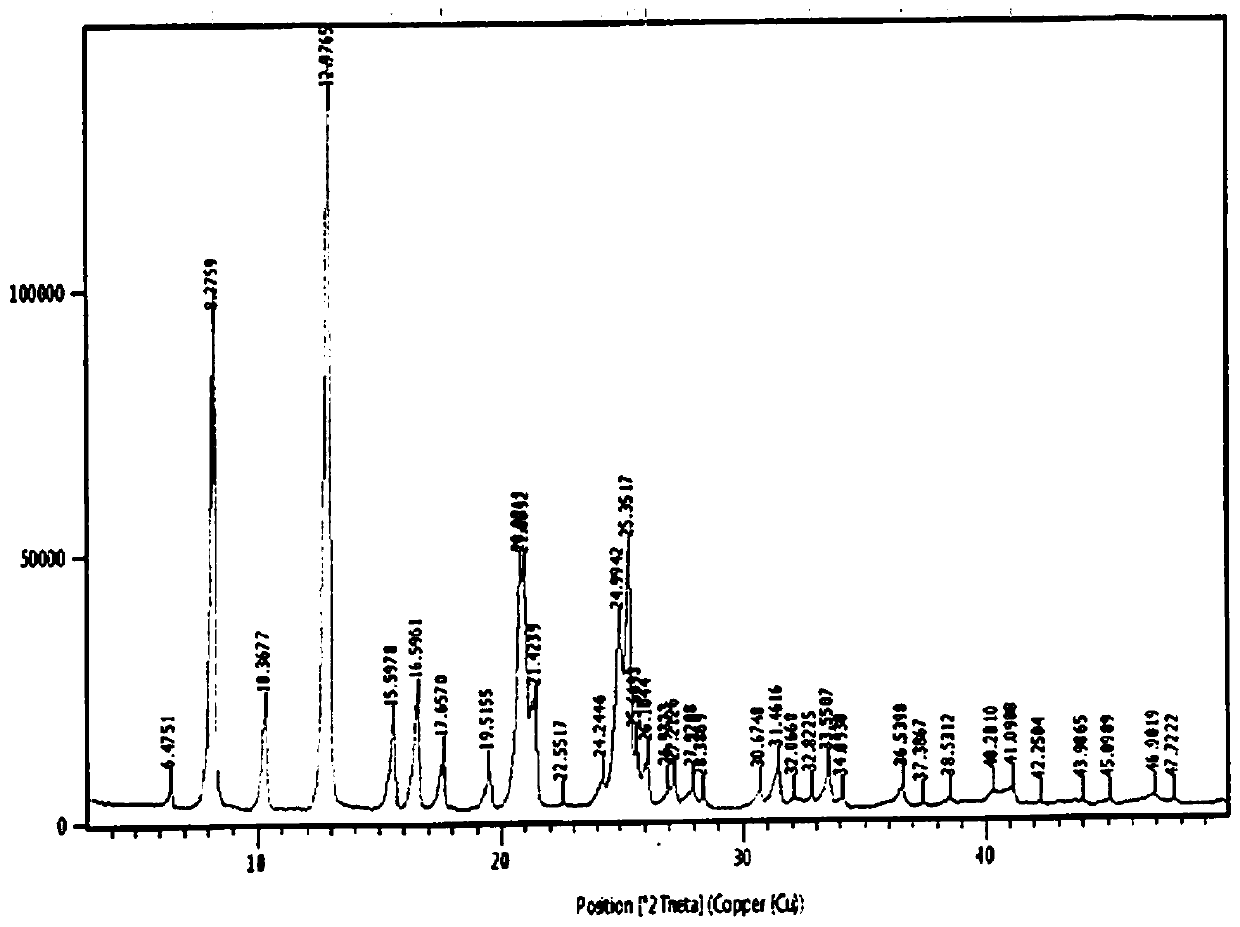
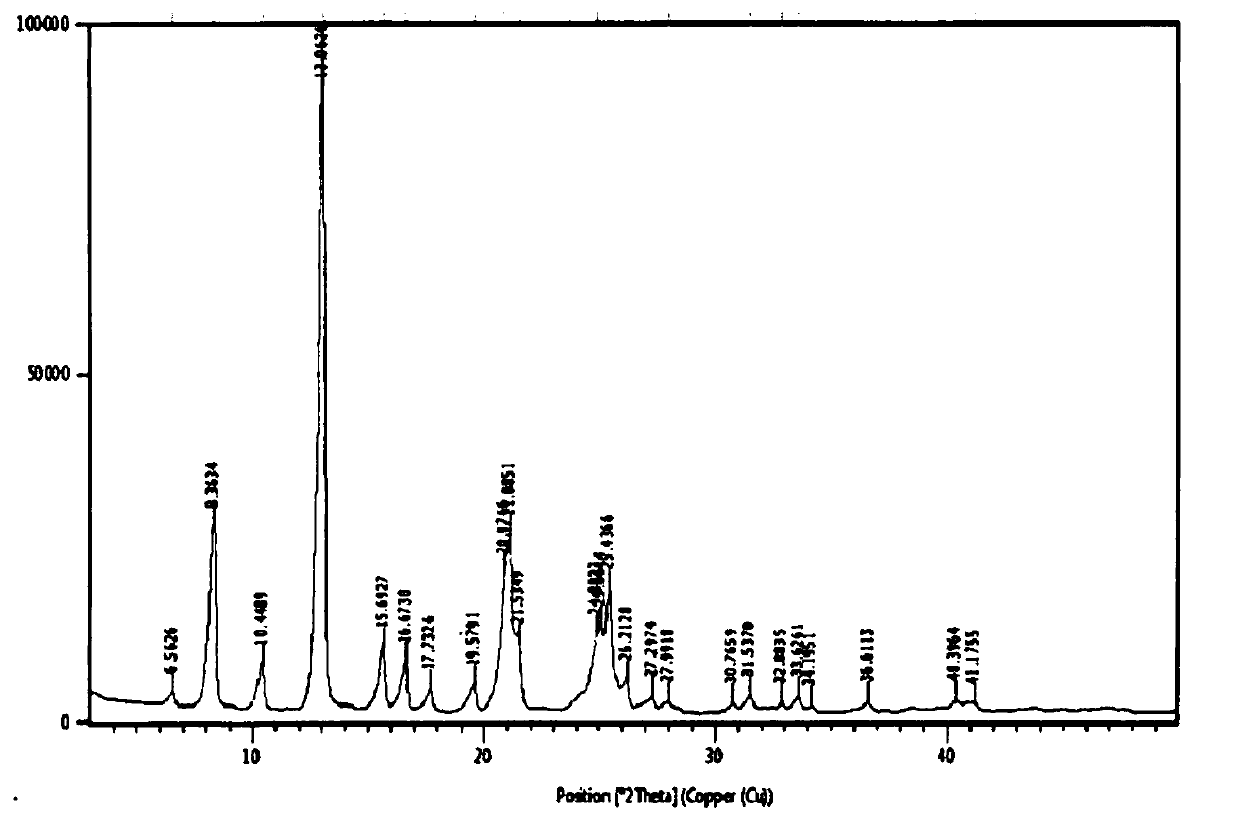
Image
Examples
Embodiment 1
[0063]
[0064] Preparation Process:
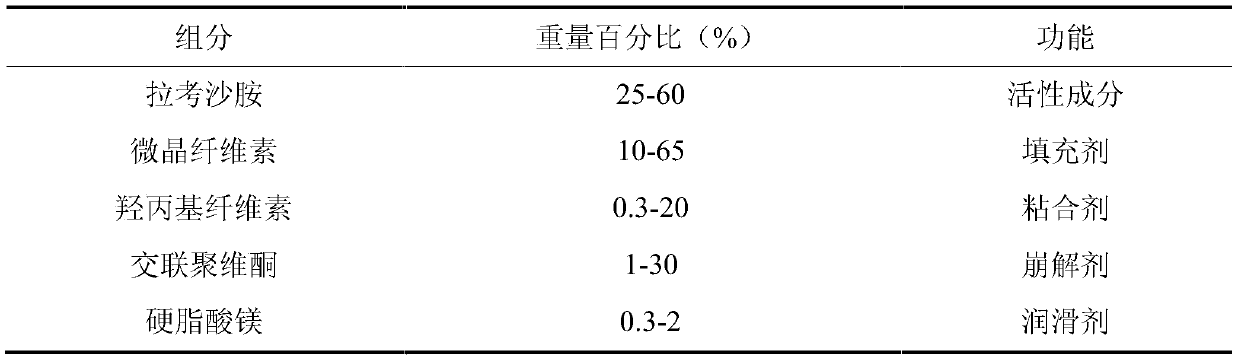
[0065] (1) Lacosamide is mixed with pharmaceutical excipients microcrystalline cellulose, crospovidone, and hydroxypropyl cellulose in a wet granulation pot to obtain a premix; add purified water to the resulting premix Medium-made soft materials, the speed of the stirring blade is 200rpm, the speed of the cutter is 1500rpm, and the granulation time is 360s;
[0066] (2) passing the soft material obtained in step (1) through a 4-20 mesh screen to obtain wet particles;
[0067] (3) The wet granules obtained in the step (2) are dried in a fluidized bed until the weight loss on drying of the material is less than or equal to 3.0%, and the obtained material is passed through a 10-30 mesh screen to obtain dry granules, and then magnesium stearate is added for total mixing , have mixed particles;
[0068] (4) The mixed granules obtained in step (3) are compressed by a rotary tablet press, and II coating powder is used for coating, and th...
Embodiment 2
[0070]
[0071] Preparation process: with embodiment 1
Embodiment 3
[0073]
[0074] Preparation process: with embodiment 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
| shading coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com