Gel sustained-release agent for treating otitis externa and preparation method thereof
A technology of otitis externa and slow-release agent, which is applied in the field of pharmaceuticals and can solve problems such as high irritation, difficulty in coating, and short drug effect time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0113] The application also relates to a preparation method for the gel sustained release agent for the treatment of otitis externa, comprising the steps of:
[0114] Step 1: adding polyvinyl alcohol to purified water, and heating in a water bath until the polyvinyl alcohol is completely dissolved, to obtain an aqueous solution of polyvinyl alcohol;
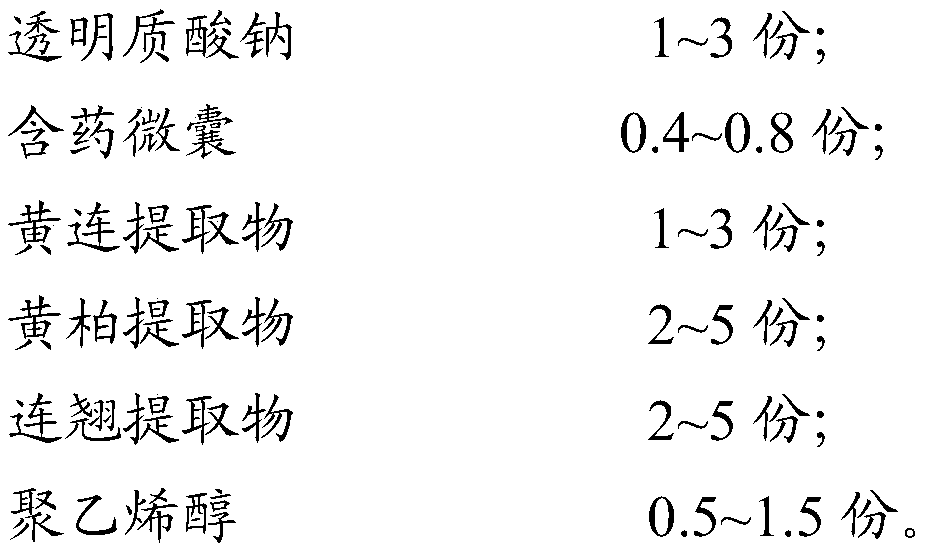
[0115] Step 2: adding sodium hyaluronate into polyvinyl alcohol aqueous solution, and mixing evenly to obtain solution A;
[0116] Step 3: Add the drug-containing microcapsules, Coptidis Rhizoma extract, Phellodendron Phellodendron extract, and Forsythia extract into solution A, and mix evenly to obtain solution B;
[0117] Step 4: dissolving the preservative in the aqueous solution to obtain an aqueous solution of the preservative;
[0118] Step 5: Add the preservative aqueous solution into the solution B, and stir evenly to obtain a gel slow-release preparation for treating otitis externa.
[0119] In step 1, polyvinyl alcoho...
Embodiment 1
[0126] Add 0.2g of polyvinyl alcohol into purified water, heat in a water bath at 60°C until the polyvinyl alcohol is completely dissolved, and mix well to obtain an aqueous solution of polyvinyl alcohol; add 0.5g of sodium hyaluronate (molecular weight: 40wDa) into polyvinyl alcohol In the aqueous solution, mix evenly to obtain solution A; add 0.2 g of drug-containing microcapsules, 0.5 g of Coptidis Rhizoma extract, 1 g of Phellodendron Phellodendron extract, and 1 g of Forsythia extract to solution A, and mix evenly to obtain solution B; Dissolve 0.08g of potassium sorbate in an aqueous solution to obtain an aqueous solution of potassium sorbate; add the aqueous solution of potassium sorbate to solution B and stir evenly to obtain the gel slow-release preparation for the treatment of otitis externa, with specific parameters such as Table 1 shows.
[0127] Wherein said drug-containing microcapsule is prepared by the following method: take gelatin, add an appropriate amount o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com