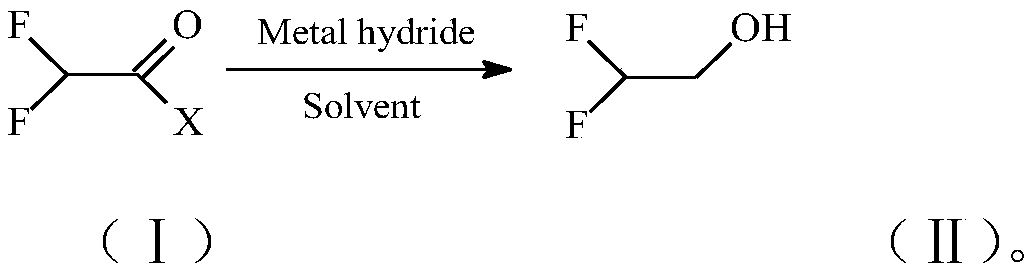
Preparation method of 2,2-difluoroethanol
A technology of difluoroethanol and difluoroacetyl halide is applied in the field of preparing 2,2-difluoroethanol, can solve the problems of high cost, low reaction yield, low yield and the like, achieves mild reaction conditions, simple process steps, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 22.6g (0.6mol) of NaBH to a 1000ml reactor with magnetic stirring, thermocouple and cooling coil 4 , then add 200ml of CH 3 Oh. Stir and keep the temperature of the reactor at about -10°C, slowly feed 98.0g (1.0mol) of CF into the reactor 2 HCOF, keep the reaction temperature at -10~0℃, wait for CF 2 After the addition of HCOF, continue to stir for 1.0 h, add hydrochloric acid to adjust the pH value of the still liquid to 3-4, and terminate the reaction. The still liquid was subjected to a rectification operation to obtain the product 2,2-difluoroethanol with a yield of 96.1%.
Embodiment 2
[0044] Add 11.4g (0.3mol) of LiAlH to a 1000ml reactor with magnetic stirring, thermocouple and cooling coil 4 and 300ml of diethylene glycol dimethyl ether, stir and keep the still temperature of the reaction kettle at about -10°C. Slowly feed 58.8g (0.6mol) of CF into the reactor 2HCOF, keep the kettle temperature at about -10~0℃, wait for CF 2 After the addition of HCOF, continue to stir for 0.5h, add hydrochloric acid to adjust the pH value of the kettle liquid to 2-3, and terminate the reaction. The still liquid was subjected to a rectification operation to obtain the product 2,2-difluoroethanol with a yield of 97.8%.
Embodiment 3
[0046] NaBH 4 and substrate CF 2 The molar ratio of HCOF was changed to 0.5:1, and all the other operating conditions were the same as in Example 1. After the reaction, the still liquid was rectified to obtain the product 2,2-difluoroethanol with a yield of 91.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com