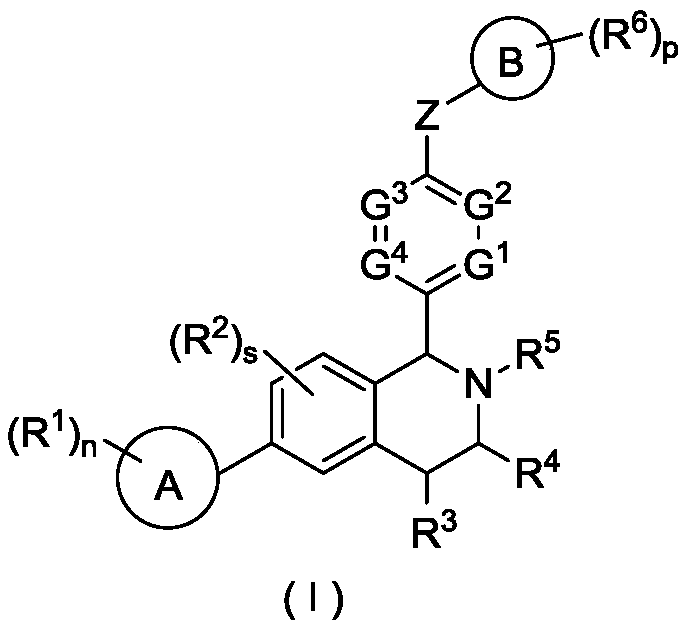
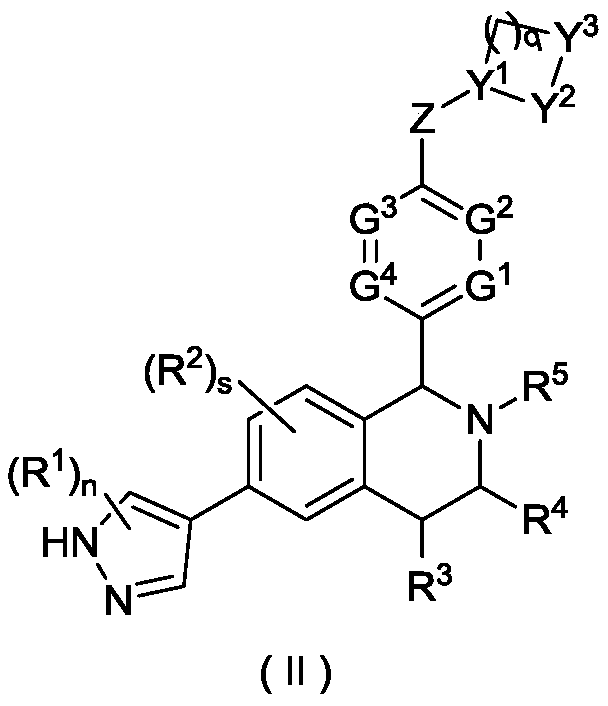
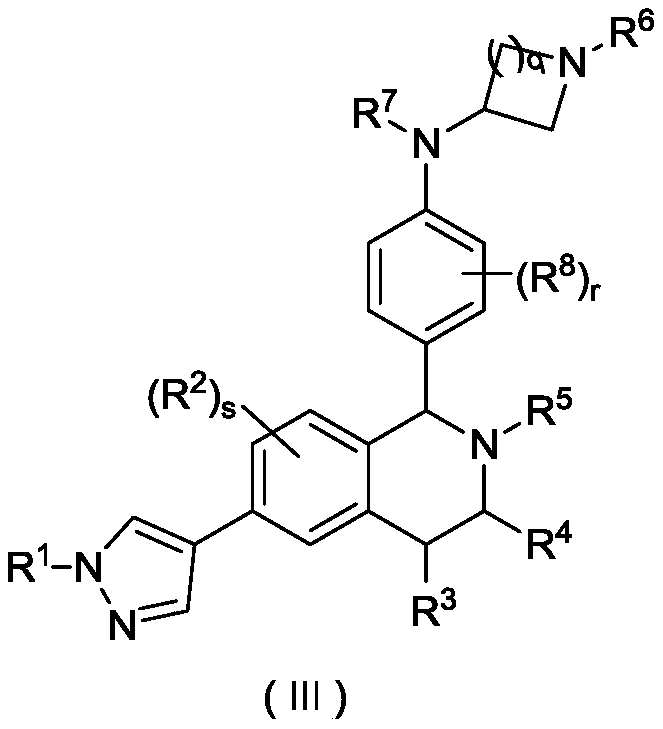
Tetrahydroisoquinoline derivative, and preparation method and medical application thereof
A technology of compounds and mixtures, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0398] N-(3,5-difluoro-4-((1S,3R)-3-methyl-6-(1H-pyrazol-4-yl)-2-(2,2,2trifluoroethyl) -1,2,3,4-tetrahydroisoquinolin-1-yl)phenyl)-1-(3-fluoropropyl)azetidinyl-3-amine 1
[0399]
[0400]
[0401] first step
[0402] (R)-tert-butyl(1-(3-(benzyloxy)phenyl)propyl-2-yl)carbamate 1c
[0403] Dissolve benzyloxy-3-bromobenzene 1a (4.00g, 15.20mmol) in tetrahydrofuran (45mL), under argon protection, stir well, add n-butyllithium (15.20mmol, 9.5mL, 1.6M), -78°C Stir for 30 minutes. Then (R)-4-methyl-1,2,3-oxathiazolidine-3-carboxylic acid tert-butyl ester 2,2-dioxide 1b (3.60g, 15.17mmol, using the patent application "WO2016162325, implemented (prepared by the method disclosed in Example 1") was dissolved in 20 mL of tetrahydrofuran, the above solution was added to the reaction solution, and the reaction was stirred at -78°C for 0.5 hours. When the temperature was slowly raised to 0°C, the reaction was stopped after stirring in an ice bath for 30 minutes. The reaction was q...
Embodiment 2
[0451] N-(3,5-difluoro-4-((1S,3R)-3-methyl-6-(1-methyl-1H-pyrazol-4-yl)-2-(2,2,2 -Trifluoroethyl)-1,2,3,4-tetrahydroisoquinolin-1-yl)phenyl)-1-(3-fluoropropyl)azetidinyl-3-amine 2
[0452]
[0453] Using the synthetic route of compound 1 in Example 1, the raw material compound 1r was replaced by the raw material compound 1-methyl-4-pyrazole boronic acid pinacol ester 2r (94mg, 451.78μmol), anhydrous sodium carbonate (56mg, 528.36umol ), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (17mg, 23.23umol) and compound 1q (140mg, 225.97umol) were reacted to obtain the title compound 2 (60mg), Yield: 39%.
[0454] MS m / z(ESI):552.2[M+1]
[0455] 1 H NMR (400MHz, CD 3 OD)7.91(s,1H),7.77(s,1H),7.26(s,1H),7.20(d,1H),6.71(d,1H),6.18-6.12(m,2H),5.18(s, 1H),4.74-4.64(m,1H),4.62(t,1H),4.50(t,1H),4.48-4.34(m,2H),4.22-4.12(m,1H),3.97-3.93(m, 1H),3.90(s,3H),3.58-3.50(m,1H),3.48-3.39(m,2H),3.37-3.33(m,1H),3.29-3.23(m,1H),2.97-2.86( m,1H),2.65(dd,1H),2.08-1.95(m,2H),1.07(d,...
Embodiment 3
[0457] N-(4-((1S,3R)-6-(1-ethyl-1H-pyrazol-4-yl)-3-methyl-2-(2,2,2-trifluoroethyl)- 1,2,3,4-Tetrahydroisoquinolin-1-yl)-3,5-difluorophenyl)-1-(3-fluoropropyl)azetidinyl-3-amine
[0458]
[0459] Using the synthetic route of compound 1 in Example 1, the raw material compound 1r was replaced by the raw material compound 1-ethyl-4-pyrazole borate pinacol ester 3r (100 mg, 450.27 μmol), anhydrous sodium carbonate (56 mg, 528.36 μmol ), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (17mg, 23.23umol) and compound 1q (140mg, 225.97umol) were reacted to obtain the title compound 3 (40mg), Yield: 31%.
[0460] MS m / z(ESI):566.3[M+1]
[0461] 1 H NMR (400MHz, CD 3 OD)7.93(s,1H),7.77(s,1H),7.28(s,1H),7.21(d,1H),6.72(d,1H),6.07(d,2H),5.15(s,1H) ,4.52(t,1H),4.41(t,1H),4.19(q,2H),4.10-4.04(m,1H),3.86-3.82(m,2H),3.55-3.51(m,1H),3.38 -3.34(m,1H),3.28-3.20(m,1H),3.07-3.03(m,2H),2.98-2.87(m,1H),2.70(t,2H),2.63(dd,1H),1.84 -1.71(m,2H),1.47(t,3H),1.07(d,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com