Preparation method of S-adenosylhomocysteine-ovalbumin conjugate
A technology for homocysteine and adenosine, which is applied in the field of preparation of S-adenosyl homocysteine-chicken ovalbumin conjugates, can solve the problem of complex process, small amount of coupling products and easy coupling agent. Overdose and other problems, to achieve the effect of simple process operation, high yield of conjugates, and good activity of conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
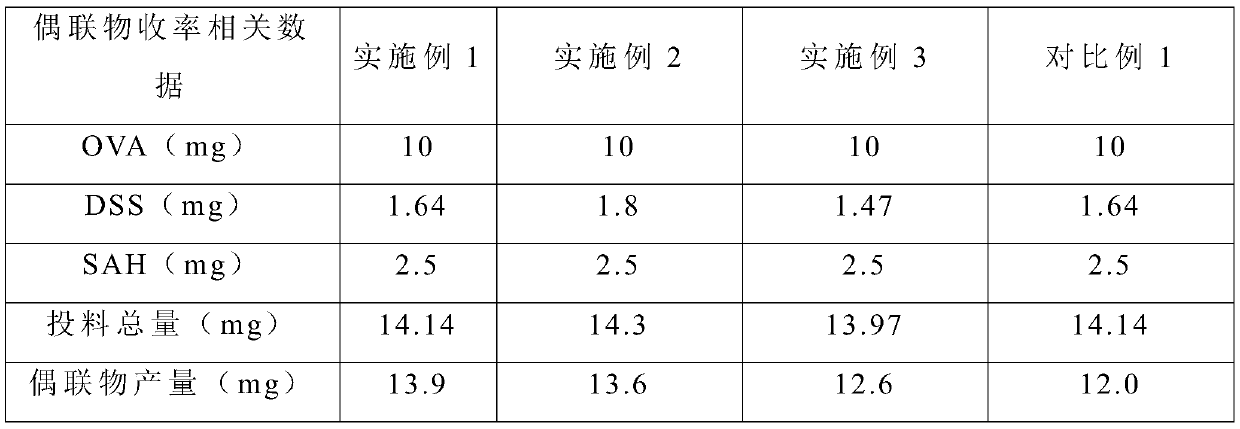
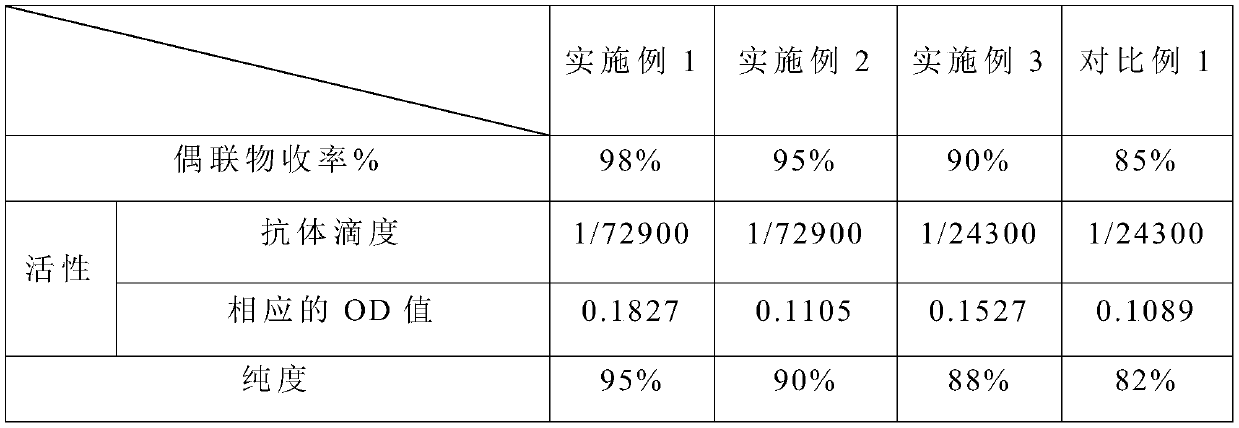
Embodiment 1
[0029] This embodiment provides a preparation method of S-adenosylhomocysteine-chicken ovalbumin conjugate, the preparation method comprising the following steps:
[0030] (1) Activate chicken ovalbumin with suberic acid disuccinimide in dimethylformamide, the activation temperature is 37°C, and the activation time is 30min; wherein the chicken ovalbumin and the suberic acid di The molar ratio of succinimide is 1: 20;
[0031] (2) The mixture after the activation of step (1) is dialyzed successively with two dialysates, centrifuged at 5000rpm for 30s, and the precipitate is discarded to obtain the supernatant, and the concentration of the discarded supernatant is measured with an ultraviolet spectrophotometer; wherein the pH value is used first Dialyze for 7.4 phosphate buffer solution for 15 minutes, and then use sodium borate buffer solution with a pH value of 9.8 for 15 minutes;
[0032] (3) Add S-adenosylhomocysteine to the supernatant obtained in step (2), add new disu...
Embodiment 2
[0036] This embodiment provides a preparation method of S-adenosylhomocysteine-chicken ovalbumin conjugate, the preparation method comprising the following steps:
[0037] (1) Activate chicken ovalbumin with suberic acid disuccinimide in dimethylformamide, the activation temperature is 37°C, and the activation time is 30min; wherein the chicken ovalbumin and the suberic acid di The molar ratio of succinimide is 1: 22;
[0038] (2) The mixture after the activation of step (1) is dialyzed successively with two dialysates, centrifuged at 5000rpm for 30s, and the precipitate is discarded to obtain the supernatant, and the concentration of the discarded supernatant is measured with an ultraviolet spectrophotometer; wherein the pH value is used first Dialyze for 7.4 phosphate buffer solution for 12 minutes, and then use sodium borate buffer solution with a pH value of 9.8 for 12 minutes;
[0039] (3) Add S-adenosylhomocysteine to the supernatant obtained in step (2), add new disu...
Embodiment 3
[0043] This embodiment provides a preparation method of S-adenosylhomocysteine-chicken ovalbumin conjugate, the preparation method comprising the following steps:
[0044](1) Activate chicken ovalbumin with suberic acid disuccinimide in dimethylformamide, the activation temperature is 37°C, and the activation time is 35min; wherein the chicken ovalbumin and the suberic acid di The molar ratio of succinimide is 1: 18;
[0045] (2) The mixture after the activation of step (1) is dialyzed successively with two dialysates, centrifuged at 5000rpm for 30s, and the precipitate is discarded to obtain the supernatant, and the concentration of the discarded supernatant is measured with an ultraviolet spectrophotometer; wherein the pH value is used first Dialyze for 7.4 phosphate buffer solution for 10 minutes, and then use sodium borate buffer solution with a pH value of 9.8 for 10 minutes;
[0046] (3) Add S-adenosylhomocysteine to the supernatant obtained in step (2), add new disuc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com