Application of three compounds in terminalia in preparation of anti-inflammatory drug and preparation method of compounds
A compound and drug technology, applied in the field of medicine, can solve the problems of application research and reporting of anti-inflammatory activity of uncompounded compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
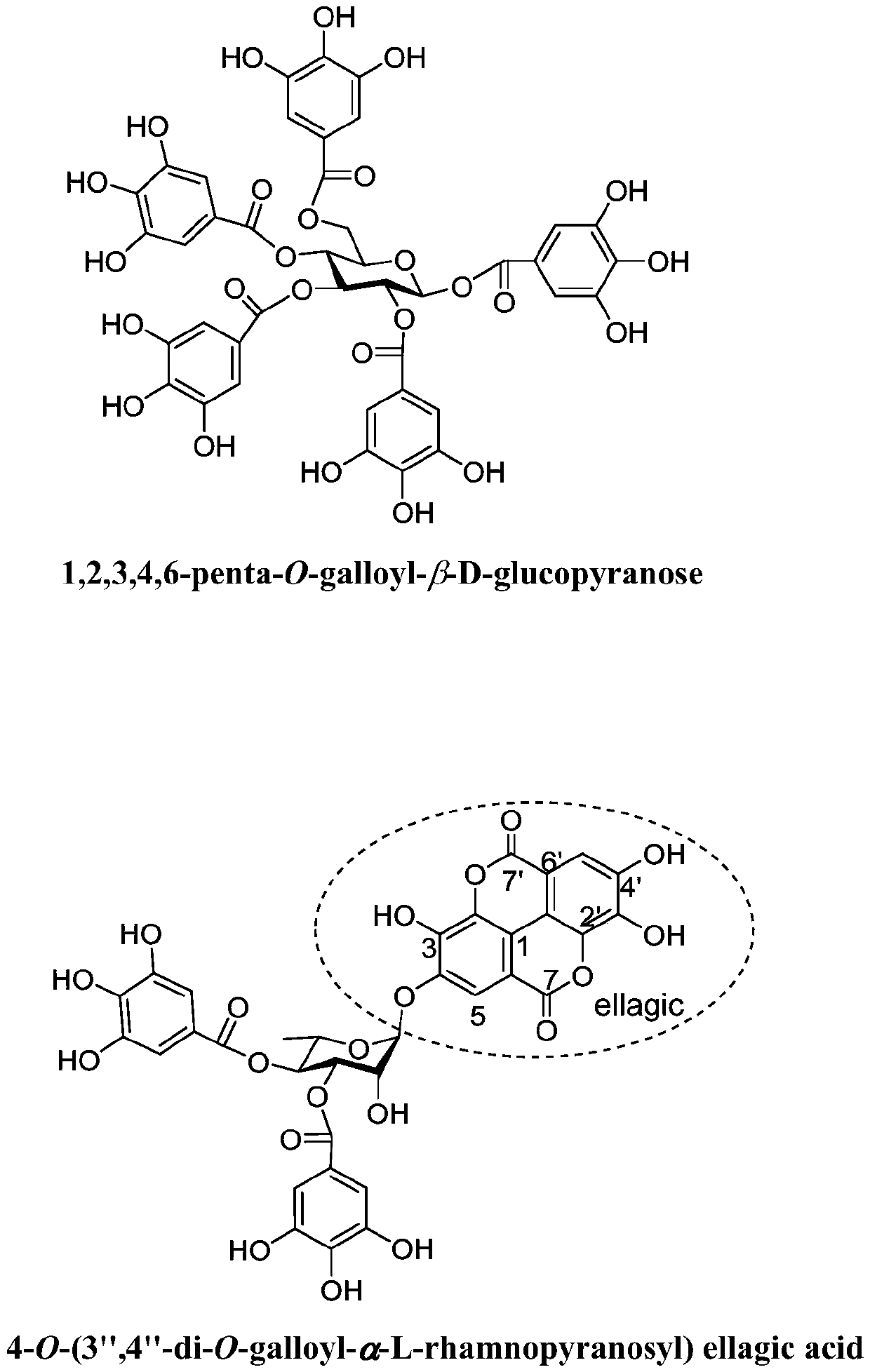
[0025] Preparation of 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose and its anti-inflammatory pharmaceutical composition, and its application in medicine.
[0026]
[0027] 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose
[0028] Step 1: Preparation of 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose:
[0029] 50kg Yongde produce the fresh fruit of Myrobalan chebula, and use 3 times the volume of 70% acetone aqueous solution to extract by cold soaking at room temperature for 3 times, each time for 5 days, filter, and the filtrate is concentrated under reduced pressure at 50°C to remove the organic solvent. The remaining aqueous solution was subjected to DiaionHP-20 column chromatography, and gradient elution was carried out with a methanol / water volume ratio of 35% and 60% solution to obtain Fr. 35 , Fr. 60 . Fr. 35 On Sephadex LH-20 chromatography column, use MeOH / H 2 O volume ratio 80% and 90% elution, wherein methanol / water volume ratio 90% elution part carries out MCI-gel CHP...
Embodiment 2
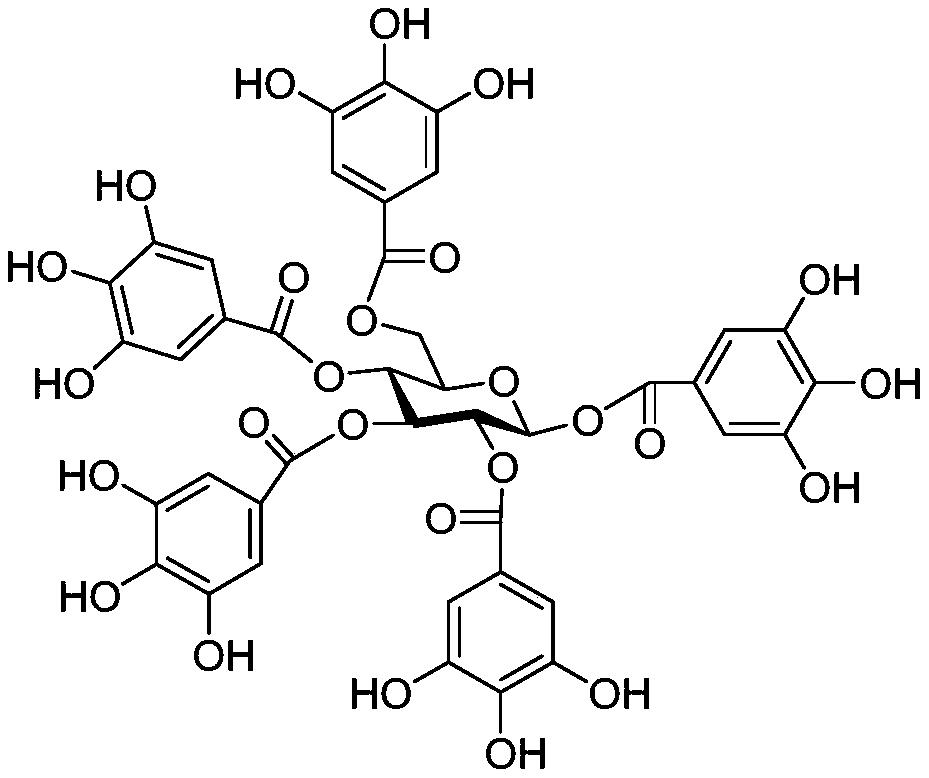
[0033] Preparation of 4-O-(3", 4"-di-O-galloyl-α-L-rhamnopyranosyl) ellagic acid, its anti-inflammatory pharmaceutical composition, and its application in medicine.
[0034]
[0035] 4-O-(3”,4”-di-O-galloyl-α-L-rhamnopyranosyl) ellagic acid
[0036] Step 1: Preparation of 4-O-(3”,4”-di-O-galloyl-α-L-rhamnopyranosyl) ellagic acid
[0037] 50kg Yongde produce the fresh fruit of Myrobalan chebula, and use 3 times the volume of 70% acetone aqueous solution to extract by cold soaking at room temperature for 3 times, each time for 5 days, filter, and the filtrate is concentrated under reduced pressure at 50°C to remove the organic solvent. The remaining aqueous solution was subjected to DiaionHP-20 column chromatography, and gradient elution was carried out with a methanol / water volume ratio of 35% and 60% solution to obtain Fr. 35 , Fr. 60 . Fr. 35 On Sephadex LH-20 chromatography column, use MeOH / H 2 O volume ratio 80% and 90% elution, wherein methanol / water volume ratio...
Embodiment 3
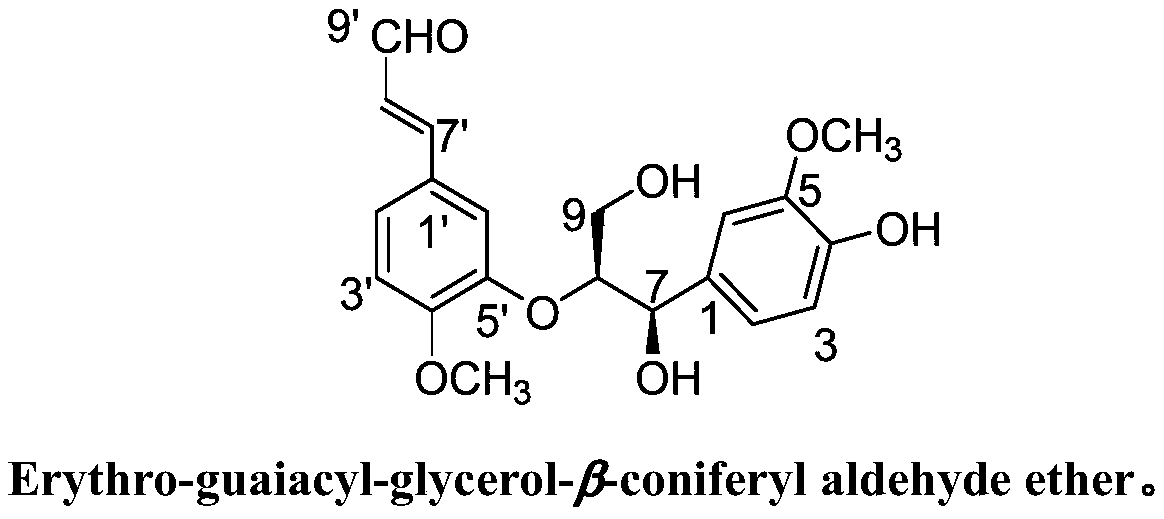
[0041] Preparation of Erythro-guaiacyl-glycerol-β-coniferyl aldehyde ether, its anti-inflammatory pharmaceutical composition, and its application in medicine.
[0042]
[0043] Erythro-guaiacyl-glycerol-β-coniferyl aldehyde ether
[0044] Step 1: Preparation of Erythro-guaiacyl-glycerol-β-coniferyl aldehyde ether
[0045] 50kg Yongde produce the fresh fruit of Myrobalan chebula, and use 3 times the volume of 70% acetone aqueous solution to extract by cold soaking at room temperature for 3 times, each time for 5 days, filter, and the filtrate is concentrated under reduced pressure at 50°C to remove the organic solvent. Under the detection guidance of thin-layer chromatography, the remaining aqueous solution was subjected to Diaion HP-20 column chromatography, and gradient elution was carried out with a methanol / water volume ratio of 35% and 60% solution to obtain Fr. 35 , Fr. 60 . Fr. 60 The MCI-gel CHP20P chromatography column was eluted with a solution with a volume ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com