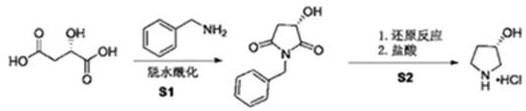
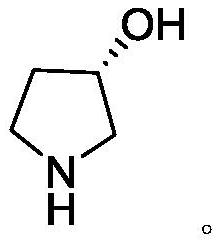
A kind of synthetic method of (s)-3-pyrrolidinol hydrochloride
A technology of pyrrolidinol and synthetic method, applied in the direction of organic chemistry, etc., can solve the problems of long steps, high reaction temperature, consumption of iodine simple substance, etc., achieve cleanliness and high efficiency of functional group conversion, reduce reaction temperature, and increase yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of (3S)-N-benzyl-3-hydroxypyrrolidine-2,5-dione
[0025] L-malic acid (100g, 1eq) was added to a 1L bottle with mechanical stirring, added to methanol (230mL), stirred and dissolved, and benzylamine (80g, 1eq) was added dropwise at a low temperature of 0-5°C. The temperature does not exceed 10°C, add dropwise for about half an hour, after the dropwise addition is complete, stir at room temperature for 3 hours, concentrate methanol until no fraction flows out, add 500mL of N-methylpyrrolidone, add 3A molecular sieves (130g), heat up to 100°C, and react 6 Hours, hot filtration, recovery of molecular sieves. The filtrate was concentrated to obtain a crude solid product, which was recrystallized from ethanol to obtain 125 g of (3S)-N-benzyl-3-hydroxypyrrolidine-2,5-dione with a yield of 82% and a purity of 98.3%.
[0026] Synthesis of (S)-3-pyrrolidinol hydrochloride
[0027] (3S)-N-Benzyl-3-hydroxypyrrolidine-2,5-dione (100g) was added to a 1L autoclave, 500mL ...
Embodiment 2
[0029] Synthesis of (3S)-N-benzyl-3-hydroxypyrrolidine-2,5-dione
[0030] L-malic acid (300g, 1eq) was added to a 3L bottle with mechanical stirring, added to methanol (700mL), stirred and dissolved, and benzylamine (240g, 1eq) was added dropwise at a low temperature of 0-5°C. The temperature does not exceed 10°C, add dropwise for about half an hour, after the dropwise addition is complete, stir at room temperature for 3 hours, concentrate methanol until no fraction flows out, add 1.5L of toluene, add 4A molecular sieve (390g), heat up to 105°C, and react for 5-6 hours , hot filtration, and recovery of molecular sieves. The filtrate was concentrated to obtain a crude solid, which was recrystallized from ethanol to obtain 390 g of (3S)-N-benzyl-3-hydroxypyrrolidine-2,5-dione with a yield of 85% and a purity of 98.5%.
[0031] Synthesis of (S)-3-pyrrolidinol hydrochloride
[0032] (3S)-N-Benzyl-3-hydroxypyrrolidine-2,5-dione (100g) was added to a 1L autoclave, 500mL of ethanol...
Embodiment 3
[0034] Synthesis of (3S)-N-benzyl-3-hydroxypyrrolidine-2,5-dione
[0035]L-malic acid (100g, 1eq) was added to a 1L bottle with mechanical stirring, added to methanol (230mL), stirred and dissolved, and benzylamine (80g, 1eq) was added dropwise at a low temperature of 0-5°C. The temperature does not exceed 10°C, add dropwise for about half an hour, after the dropwise addition is complete, stir at room temperature for 3 hours, concentrate methanol until no fraction flows out, add 500mL of toluene, add 3A molecular sieves (100g), heat up to 80°C, react for 6 hours, and filter hot , to recover molecular sieves. The filtrate was concentrated to obtain a crude solid, which was recrystallized from ethanol to obtain 114 g of (3S)-N-benzyl-3-hydroxypyrrolidine-2,5-dione, with a yield of 82% and a purity of 88.4%.
[0036] Synthesis of (S)-3-pyrrolidinol hydrochloride
[0037] (3S)-N-Benzyl-3-hydroxypyrrolidine-2,5-dione (10g) was added to the autoclave, 50mL of ethanol was added, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com