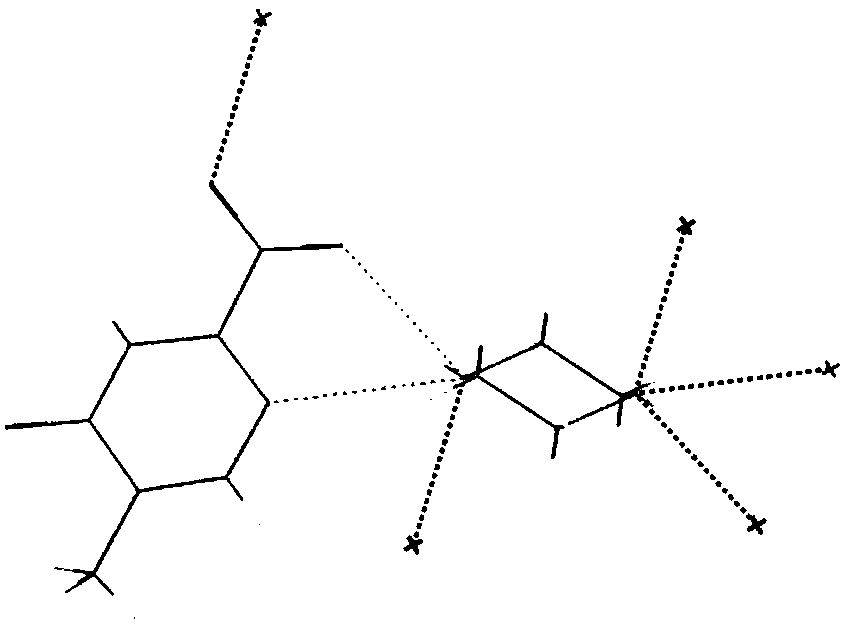
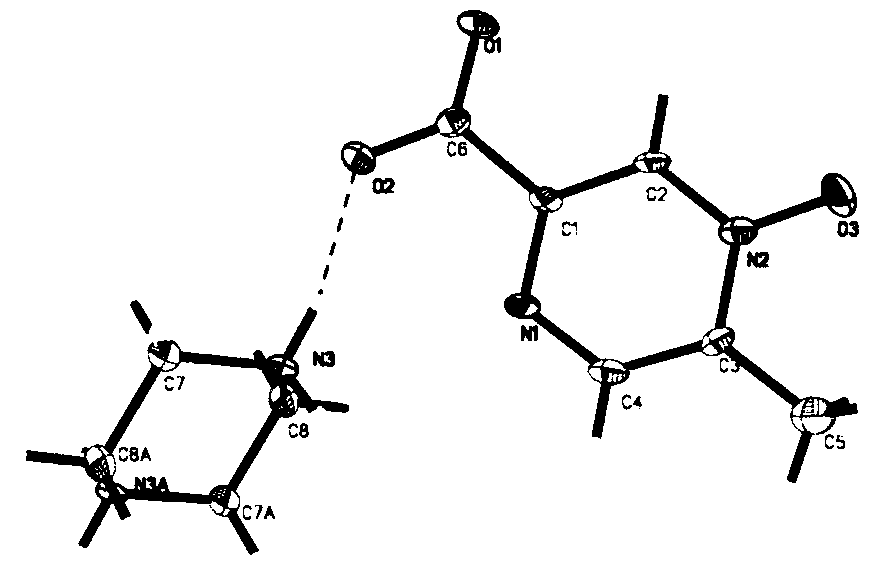
Methylpyrazine derivative-piperazine eutectic crystal
A technology for methylpyrazine and derivatives, applied in the field of methylpyrazine derivatives-piperazine co-crystals, can solve the problems of low yield, unmentioned characterization parameters, etc., and achieves high bioavailability and solid state. Good stability and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
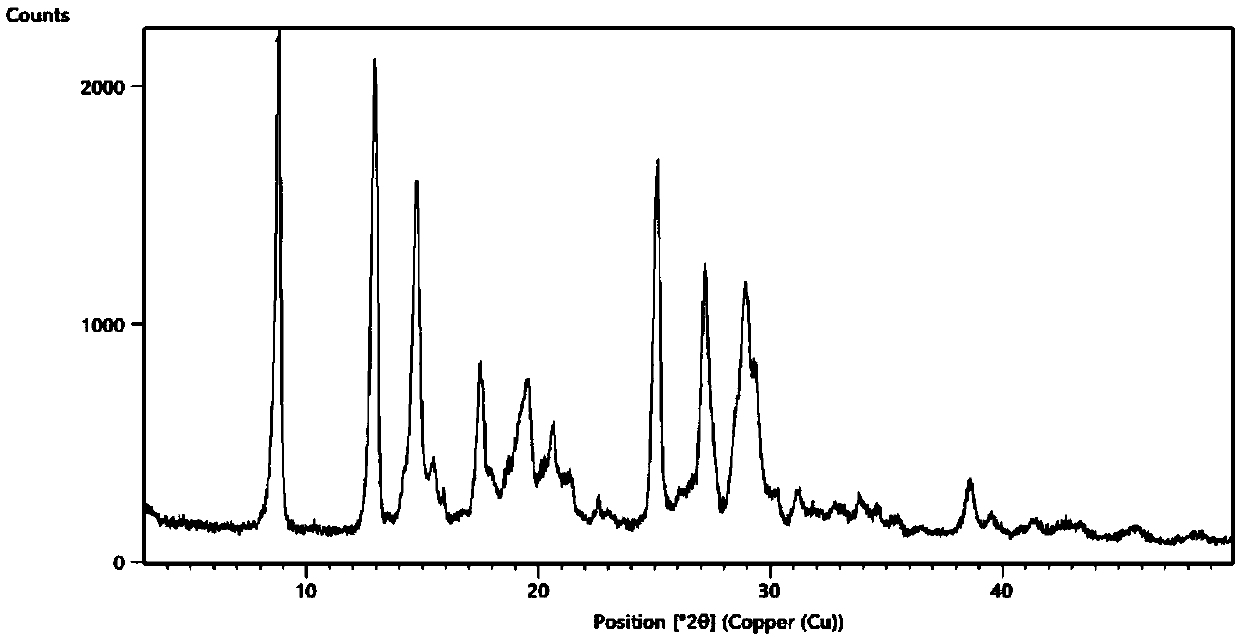
Embodiment 1
[0055] Add 68.9mg of acipimox and 38.5mg of piperazine into 8ml of methanol, heat to 60°C and stir to dissolve, reflux reaction for 10 hours, slowly cool down to 15-20°C, stand for crystallization under temperature control for 26 hours, filter , Vacuum-dried at 50°C for 8h, the acipimus-piperazine co-crystal, the yield was 95.88%, the purity was 99.94%, and the impurity I: 0.04%.
Embodiment 2
[0057] Add 65.5mg of acipimox and 38.5mg of piperazine into 5.5ml of ethanol, heat to 70°C and stir to dissolve, reflux for 11 hours, slowly lower the temperature to 20-25°C, stand for crystallization under temperature control for 28 hours, filter , vacuum-dried at 45°C for 8h, the yield of acipimus-piperazine cocrystal was 95.68%, the purity was 99.92%, and the impurity I: 0.05%.
Embodiment 3
[0059] Add 72.3mg of acipimox and 38.5mg of piperazine to 13ml of acetone, heat to 55°C and stir to dissolve, reflux reaction for 12 hours, slowly cool down to 10-15°C, stand for crystallization under temperature control for 30 hours, filter , Vacuum-dried at 50°C for 8h, acipimus-piperazine cocrystal, yield 94.81%, purity 99.90%, impurity I: 0.07%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com