Oxothiazine compound and application thereof
An oxthiazide and compound technology, applied in the field of innovative chemical drugs, can solve problems in the preclinical research stage or clinical trial stage, etc., and achieve the effects of significant drug-like properties, enhanced function, and reduced receptor inactivation rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
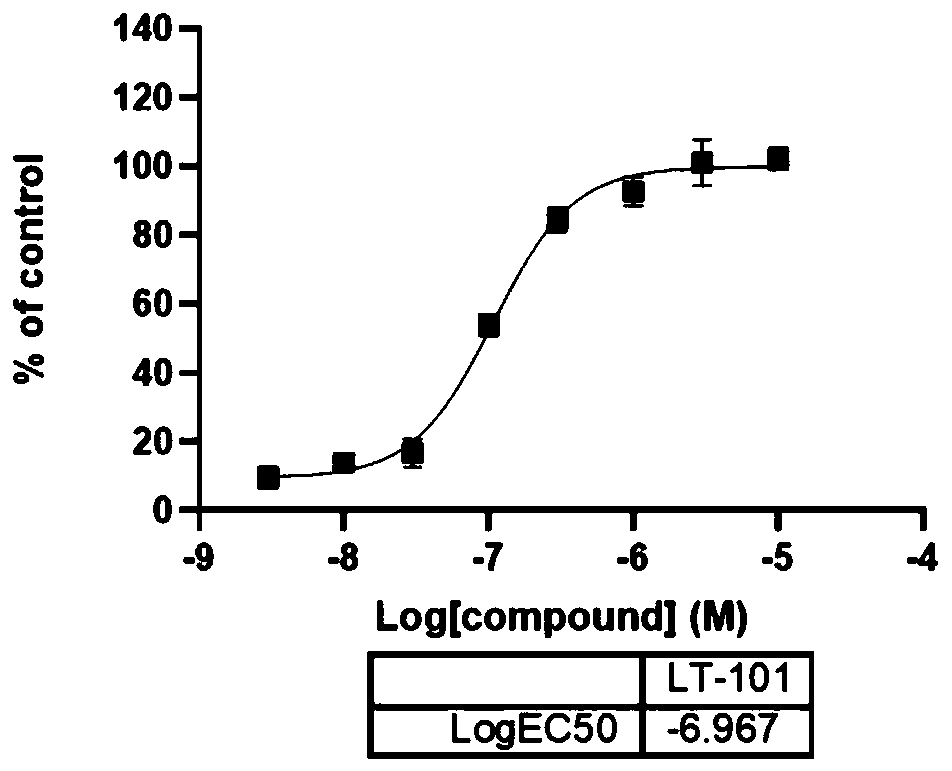
[0047] Compounds disclosed in this example: 8-(4-methoxyphenyl)-4-methyl-3,4-dihydrobenzo[e][1,2,3]oxathiazine 2,2-dioxide ( LT-101)
[0048]
[0049] Concrete synthetic route is as follows:
[0050]
[0051] The specific preparation method is as follows:
[0052] At 0°C, 3-bromo-2-hydroxyacetophenone (A01a) (6.0g, 28mmol) was dispersed in 60ml of dimethylacetamide DMA, and then aminosulfonyl chloride (10g, 86.5 mmol). The reaction system was stirred and slowly raised to room temperature until the reaction was complete. After the reaction, add water to precipitate a solid, filter and wash the filter cake with water and ethyl acetate respectively, and obtain the compound 8-bromo-4-methylbenzo[e][1,2,3]oxathia with a yield of 60%. Azine 2,2-dioxide (A01b).
[0053]Disperse A01b (2.75g, 10mmol) in 50ml of MeOH solvent system, add sodium borohydride (380mg, 10.6mmol) in batches at room temperature, and continue stirring at room temperature until the reaction of raw mate...
Embodiment 2
[0057] Compounds disclosed in this example: 7-(4-methoxyphenyl)-4-methyl-3,4-dihydrobenzo[e][1,2,3]oxathiazine 2,2-dioxide ( LT-102).
[0058]
[0059] Concrete synthetic route is as follows:
[0060]
[0061] The specific preparation method is as follows:
[0062] At 0°C, 4-bromo-2-hydroxyacetophenone (B02a) (6.0g, 28mmol) was dispersed in 60ml of dimethylacetamide DMA, and then sulfamoyl chloride (10g, 86.5 mmol). The reaction system was stirred and slowly raised to room temperature until the reaction was complete. After the reaction, add water to precipitate a solid, filter and wash the filter cake with water and ethyl acetate respectively, and obtain the compound 7-bromo-4-methylbenzo[e][1,2,3]oxathia with a yield of 63%. Oxazine 2,2-dioxide (B02b).
[0063] Disperse B02b (2.75g, 10mmol) in 50ml of MeOH solvent system, add sodium borohydride (380mg, 10.6mmol) in batches at room temperature, and continue stirring at room temperature until the reaction of raw mate...
Embodiment 3
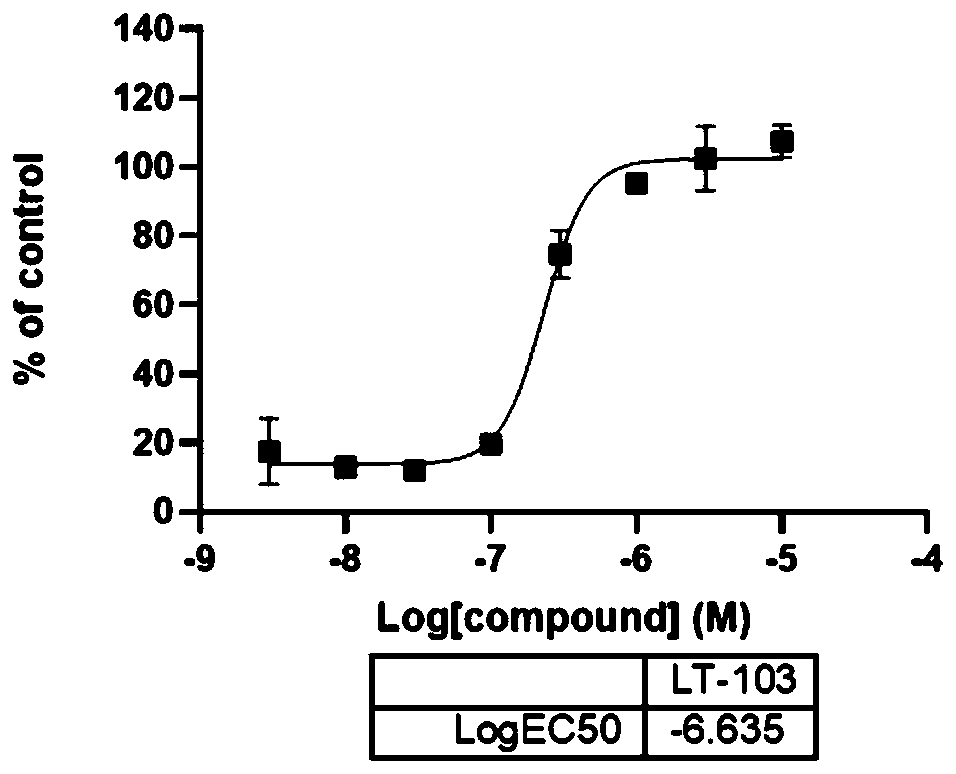
[0067] Compound disclosed in this example: 4-methyl-8-(4-phenoxyphenyl)-3,4-dihydrobenzo[e][1,2,3]oxathiazine 2,2-dioxide (LT-103)
[0068]
[0069] The synthetic route is as in Example 1, replacing "p-methoxyphenylboronic acid" with "4-phenoxyphenylboronic acid".
[0070] MS(ESI)368.1[M+H] + ; 1 H NMR (400MHz, DMSO) δ8.51(s, 1H), 7.49–7.36(m, 6H), 7.31(t, J=7.6Hz, 1H), 7.19(t, J=7.4Hz, 1H), 7.14 –7.04(m, 4H), 4.83(q, J=6.8Hz, 1H), 1.64(d, J=6.9Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com