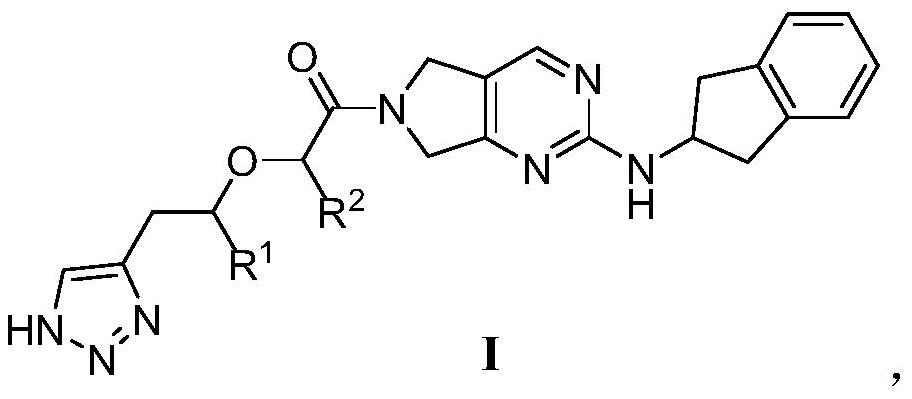
Pyrrolopyrimidine derivatives and uses thereof
A use and drug technology, applied in the field of biomedicine, can solve the problems of patients with severe specific pulmonary fibrosis not benefiting, affecting the quality of life, and unable to improve the quality of life of patients, achieving excellent liver metabolic stability and cardiac safety advantages, giving Effects of low dose and frequency of administration and good pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
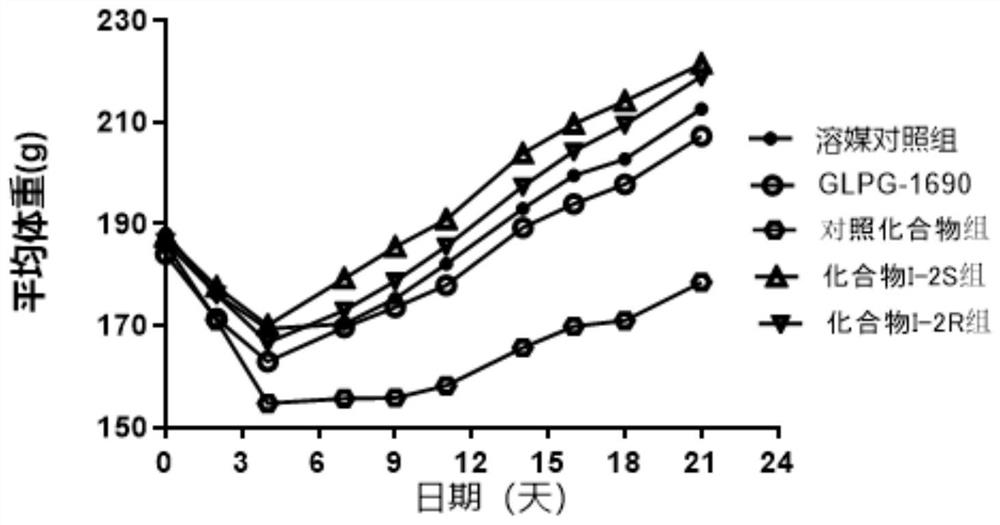
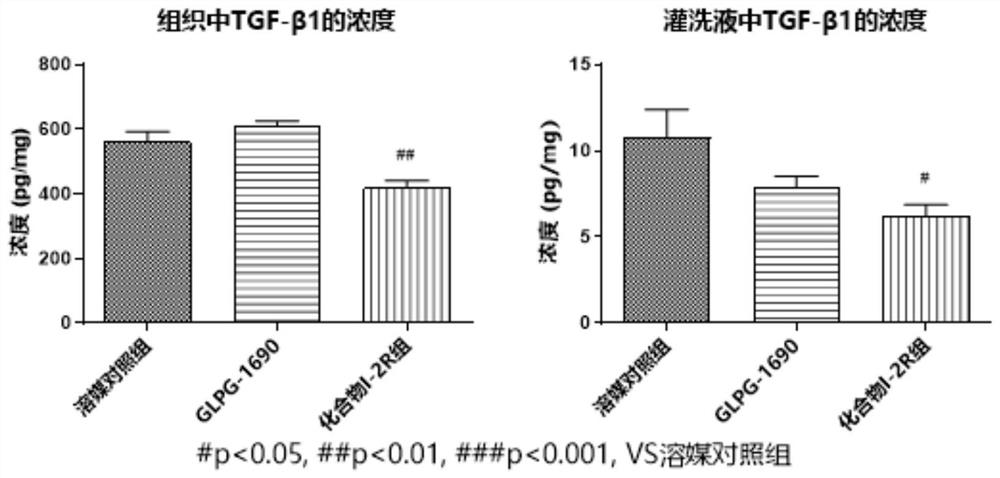
Image
Examples
Embodiment 1
[0087] Example 1: Preparation of target compound I-1
[0088] 2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((2,3-dihydro-1H-inden-2-yl)amino) -5,7-Dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)propan-1-one (target compound I-1)
[0089] 2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((2,3-dihydro-1H-inden-2-yl)amino)-5,7 -dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)propan-1-one (target compound I-1)
[0090]
[0091] The synthetic route of the target compound I-1 is as follows:
[0092]
[0093] The first step: Synthesis of tert-butyl 2-(but-3-yn-1-yloxy)propanoate (1C)
[0094] tert-butyl 2-(but-3-yn-1-yloxy)propanoate(1C)
[0095]
[0096] At 0°C, 3-butyn-1-ol (15 g, 214 mmol) was added to dichloromethane (300 mL), followed by tetrabutylammonium hydrogen sulfate (7.27 g, 21.40 mmol), sodium hydroxide (300 mL) , 3.57 mmol, 40% aq), tert-butyl 2-bromopropionate (49.2 g, 235 mmol), then slowly warmed to room temperature, stirred at room temperature for 2 hours, the reaction mixture w...
Embodiment 2
[0112] Example 2: Preparation of target compound I-1R
[0113] (R)-2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((2,3-dihydro-1H-indene-2 -yl)amino)-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)propan-1-one (target compound I-1R)
[0114] (R)-2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((2,3-dihydro-1H-inden-2-yl)amino) -5,7-dihydr o-6H-pyrrolo[3,4-d]pyrimidin-6-yl)propan-1-one (target compound I-1R)
[0115]
[0116] The synthetic route of the target compound I-1R is shown below:
[0117]
[0118] Step 1: Synthesis of (R)-2-(butyl-3-yn-1-oxy)propionic acid (2C)
[0119] (R)-2-(but-3-yn-1-yloxy)propanoic acid(2C)
[0120]
[0121] 3-Butyn-1-ol (350 mg, 5 mmol) was added to DMF (5 mL), cooled to 0 °C, NaH (400 mg, 10 mmol, 60%) was added, and stirred for 30 minutes, the starting material (S) was added at 0 °C -2-Bromopropionic acid (700 mg, 4.5 mmol), stirred at the same temperature for 5 h. Water (10 mL) was added to the reaction solution at 0°C, pH=1~2 was adjusted wit...
Embodiment 3
[0132] Example 3: Preparation of target compound I-1R and target compound I-1S
[0133](R)-2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((2,3-dihydro-1H-indene-2- yl)amino)-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)propan-1-one (target compound I-1R)
[0134] (R)-2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((2,3-dihydro-1H-inden-2-yl)amino) -5,7-dihydr o-6H-pyrrolo[3,4-d]pyrimidin-6-yl)propan-1-one (target compound I-1R)
[0135] (S)-2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((2,3-dihydro-1H-indene-2- yl)amino)-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)propan-1-one (target compound I-1S)
[0136] (S)-2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((2,3-dihydro-1H-inden-2-yl)amino) -5,7-dihydr o-6H-pyrrolo[3,4-d]pyrimidin-6-yl)propan-1-one (target compound I-1S)
[0137]
[0138] The target compounds were separated by SFC:
[0139] The racemate 2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((2,3-dihydro-1H-indene-2- yl)amino)-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)propan-1-one ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com