Immunosuppression or anti-inflammatory function enhanced PD-L1 positive mesenchymal stem cells (MSCs), induction kit and application
A PD-L1, stem cell technology, applied in anti-inflammatory agents, cell culture active agents, animal cells, etc., can solve problems such as differences in clinical efficacy, immune regulation ability or weak anti-inflammatory function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
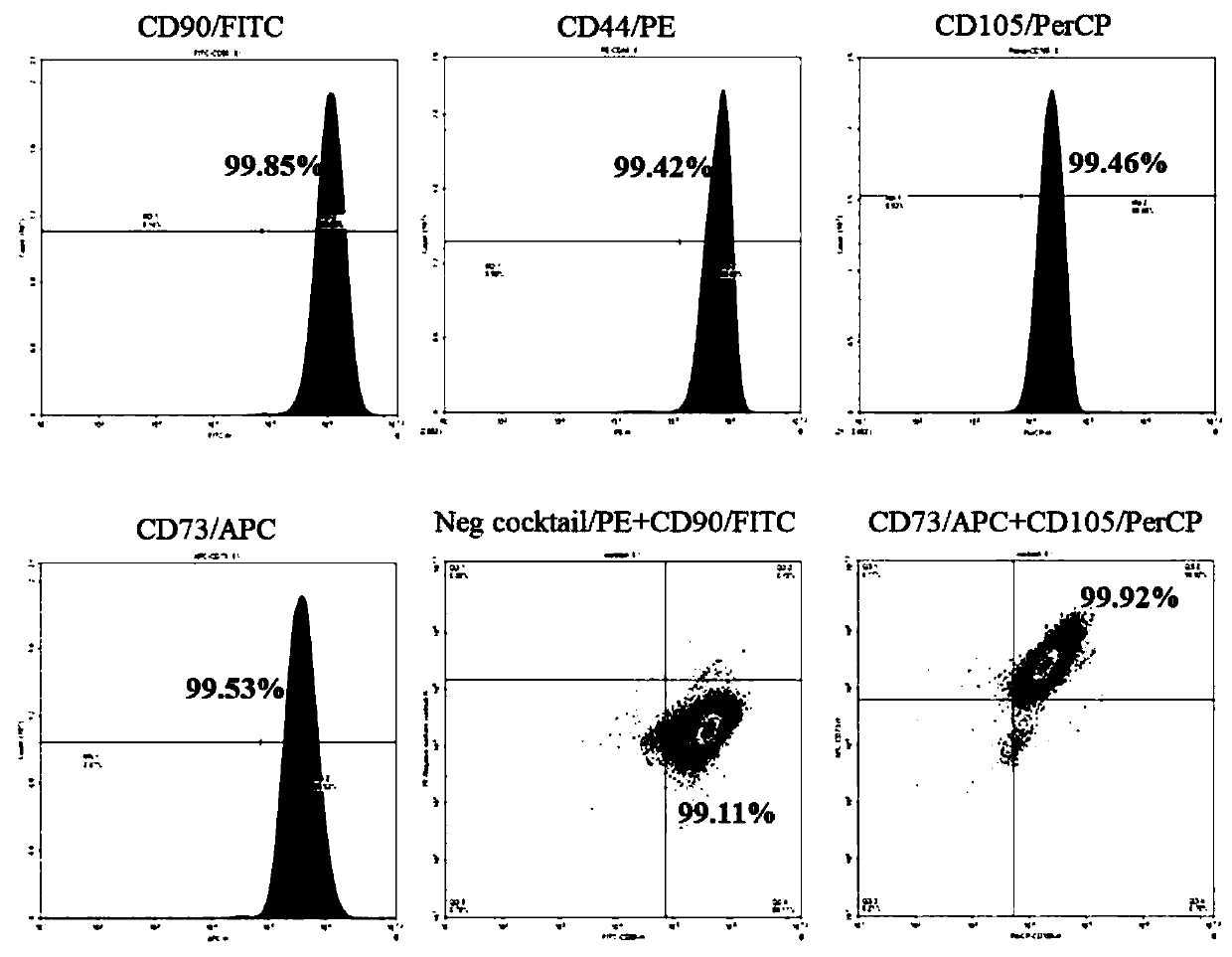
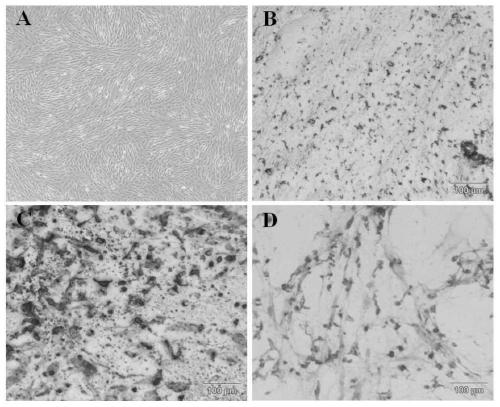
[0040] Example 1 Isolation, cultivation and identification of human umbilical cord mesenchymal stem cells
[0041] The preparation process of human umbilical cord mesenchymal stem cells was established.
[0042] Isolate, culture and identify human umbilical cord mesenchymal stem cells from neonatal umbilical cord, the specific steps are:
[0043] (1) Isolation and culture of MSCs
[0044] Isolation and culture of human umbilical cord mesenchymal stem cells (hUC-MSCs): Fresh umbilical cord samples collected under sterile conditions (the samples were from healthy puerperas who were negative for infectious diseases such as hepatitis B, hepatitis C, HIV and syphilis and had no genetic diseases) in Wash in normal saline on the ultra-clean workbench, wash the blood and other substances on the surface, transfer it to an alcohol dish to soak for about 1min, and then transfer it to a clean physiological saline dish to wash off the alcohol on the umbilical cord; and put the umbilical c...
Embodiment 2
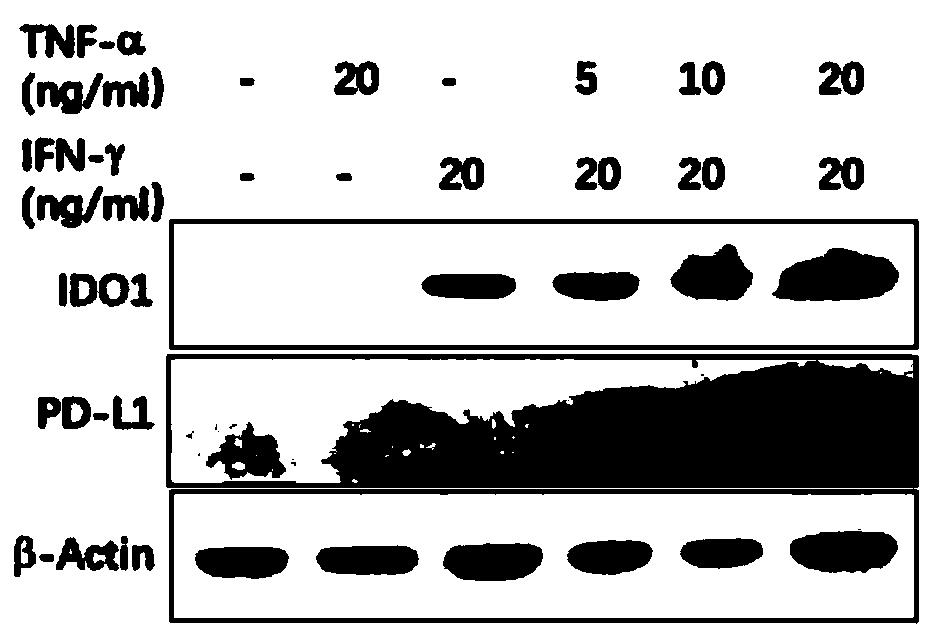
[0053] Example 2 Induction, culture and identification of human umbilical cord mesenchymal stem cells with enhanced immunosuppression or anti-inflammatory function
[0054] The hUC-MSCs prepared in Example 1 were jointly induced by IFN-γ and TNF-α to obtain hUC-MSCs with positive expression of PD-L1, immunosuppression or enhanced anti-inflammatory function. The specific steps are:
[0055] 1. Preparation of PD-L1-positive immunosuppressive or enhanced anti-inflammatory function hUC-MSCs induced in vitro
[0056] The hUC-MSCs cryopreserved cells obtained in Example 1 were resuscitated, and 4×10 4 cells / cm 2 The cell density was seeded at 75cm 2 Place the culture bottle in an incubator (37°C, 5% CO 2 incubator), when the cultured cells grew to a cell density >80%, they were digested and dissociated with TrypLE and subcultured, and the digested cells were divided into 4×10 4 cells / cm 2 The cell density was seeded at 25cm 2 culture flasks, and divide the cells into IFN-γ (0,...
Embodiment 3
[0067] Example 3 Application of human umbilical cord mesenchymal stem cells with enhanced immunosuppression or anti-inflammatory function in the treatment of mice with collagen-induced arthritis
[0068] 1. Establishment and evaluation of mouse collagen induced arthritis (CIA) model
[0069] A CIA mouse model was constructed, and the CIA mice were evaluated using the paw swelling for arthritis clinical score. Clinical arthritis was assessed using the following system: 0 points, no swelling; 1 point, mild swelling and erythema; 2 points, marked edema; 3 points, joint stiffness. Each limb is scored and aggregated to a maximum possible score of 12 per animal.
[0070] 2. Application of TNF-α combined with IFN-γ-induced immunosuppressive or anti-inflammatory enhanced hUC-MSCs in collagen-induced arthritis mouse model
[0071] 1) Protocol for treating collagen model mice with MSCs: Treat hUC-MSCs with 20ng / ml TNF-α combined with 20ng / ml IFN-γ as used in Example 2 for 24 hours, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com