Preparation method of lactate oxidase bioelectrode
A lactate oxidase and bioelectrode technology, applied in the field of bioengineering, can solve the problems of easy inactivation, difficult purification, and cannot be reused, and achieves the effects of high immobilization efficiency and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A first aspect of the present invention provides a method for preparing a lactate oxidase bioelectrode, comprising the steps of:
[0037] (1) Electrochemical oxidation treatment of Pb electrode;
[0038] (2) Use immobilized carrier to immobilize the enzyme.
[0039] In one embodiment, the electrochemical oxidation treatment of the Pb electrode comprises the steps of:
[0040] A1: The Pb electrode is placed in a mixed solution of nitric acid and potassium dichromate for electrochemical oxidation treatment;
[0041] A2: Use cyclic voltammetry to scan a circle, and perform electrochemical oxidation treatment.
[0042] In one embodiment, the mass concentration of nitric acid is 8-12%; the mass concentration of potassium dichromate is 1.5-3.5%; preferably, the mass concentration of nitric acid is 9-11%; the mass concentration of potassium dichromate 2.1-2.8%; more preferably, the mass concentration of nitric acid is 10%; the mass concentration of potassium dichromate is 2...
Embodiment 1
[0064] Embodiment 1 of the present invention provides a kind of lactate oxidase, and its preparation method is as follows:
[0065] B1: Extract the entire gene of Yarrowia, and design a pair of primers for PCR amplification of the target gene LOX to obtain the target gene sequence;
[0066] B2: Using the method of homologous recombination, using a one-step cloning kit, 37 ° C water bath for 30 minutes to connect the target gene obtained in step (1) with the starting vector pET-28a(+) to construct an expression vector;
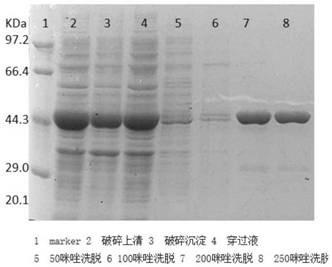
[0067] B3: Use the host strain E.coli BL21(DE3) to construct the genetically engineered strain E.coliBL21(DE3-pET-LOX) containing the LOX gene, insert the constructed LOX strain into an LB shaker flask, and culture it at 37°C for 3.5h. Then 25 μL of 50 mM lactose solution was added, cultured at 20° C. for 24 hours, and the lactate oxidase gene was expressed. Ultrasonic crushing is carried out again, the supernatant obtained by crushing is a crushed supernatant...
Embodiment 2
[0070] Embodiment 2 of the present invention provides bioelectrode material, and its preparation method is as follows:
[0071] (1) Electrochemical oxidation treatment of Pb electrode;
[0072] A1: The Pb electrode is placed in the mixed solution of nitric acid and potassium dichromate for electrochemical oxidation treatment; the mass concentration of nitric acid is 10%; the mass concentration of potassium dichromate is 2.6%; the mass concentration of nitric acid and potassium dichromate The ratio is 0.82;
[0073] A2: Use cyclic voltammetry to scan a circle for electrochemical oxidation treatment;
[0074] (2) Use the immobilized carrier to immobilize the enzyme, and the immobilization method is:
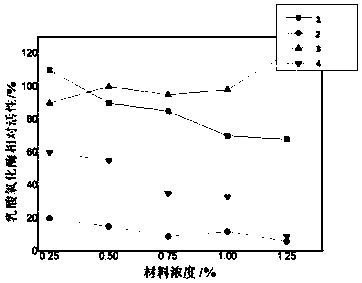
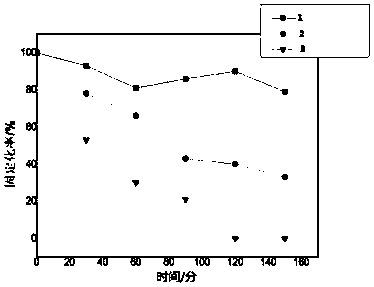
[0075] Mix 1.0 U of enzyme solution with the immobilized carrier, drop it onto the surface of the electrode obtained in step (1), and place it in the dark at 4°C until dry; the volume ratio of the enzyme solution to the immobilized carrier is 1:1;
[0076] The enzyme liquid is t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com