New crystal form of acalabrutinib and its preparation method and use
A cu-ka and material crystal technology, applied in the direction of organic chemical methods, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems affecting and affecting the clinical efficacy and safety of drugs, and achieve good physical stability, Good purification effect, good acceleration and stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0208] Example 1 The preparation method of L-ethyl lactate solvate crystal form A
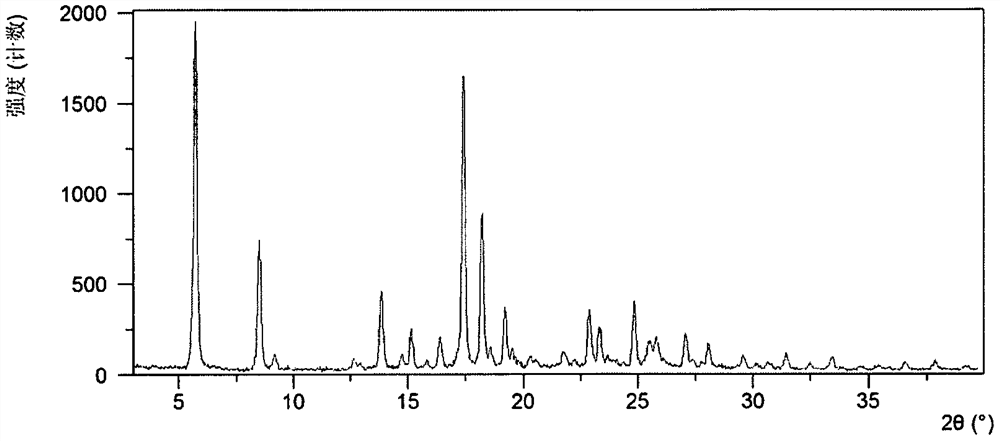
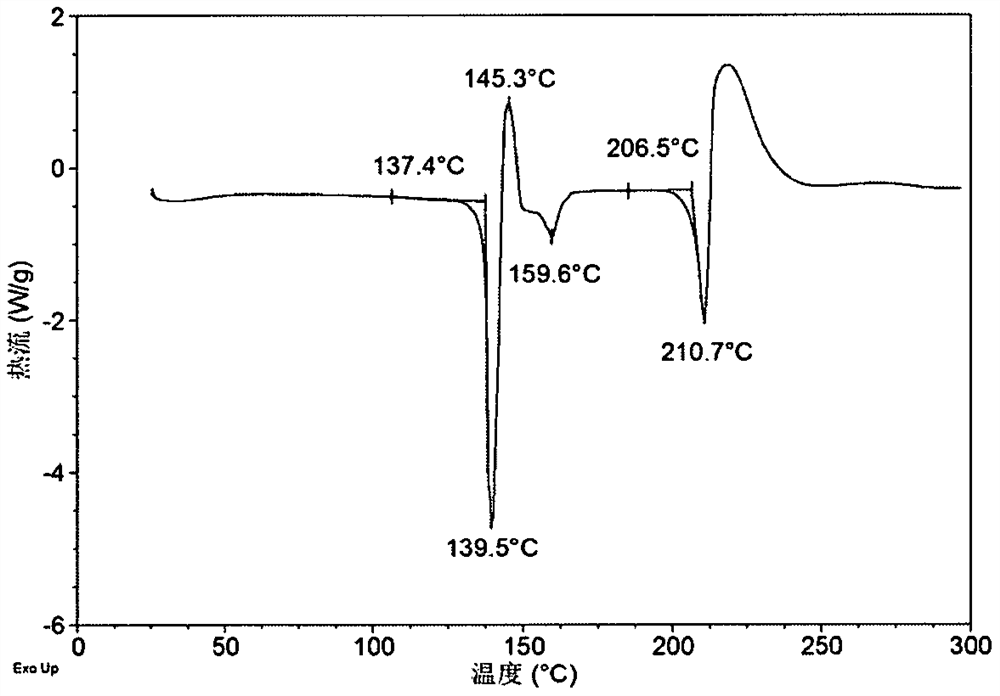
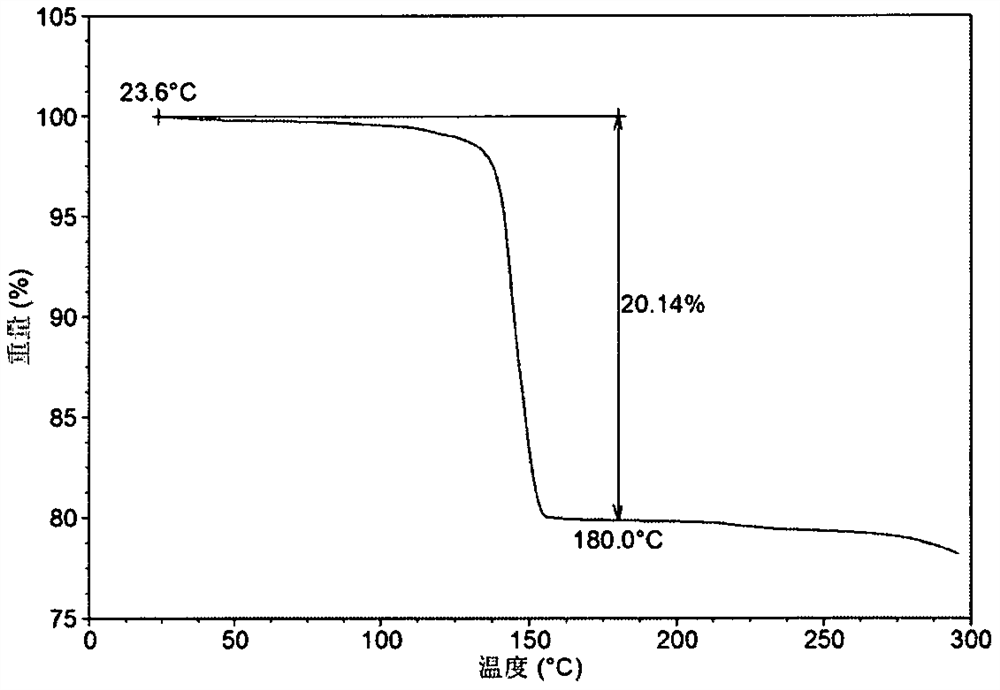
[0209] Weigh 2.50g of Acalabrutinib free base into a 100mL crystallization kettle, add 25mL of L-ethyl lactate, raise the temperature to 40°C, stir for 8 hours, cool down to 20°C, stir overnight, and separate to obtain a solid. After testing, the solid obtained in this example is L-lactate ethyl solvate crystal form A. Its XRPD is attached figure 1 , and its XRPD data sheet is shown in Table 1. Its DSC diagram is attached figure 2 , when heated to 137°C, the first endothermic peak appears, which is the desolvation endothermic peak. Its TGA diagram is attached image 3 , has a mass loss of about 20.1% when heated to 180°C. liquid hydrogen NMR Figure 4 , according to NMR data, it can be seen that L-ethyl lactate solvate crystal form A contains about 1 mole of L-ethyl lactate, and the NMR peaks around 1.25, 4.09 and 5.34 are the characteristic peaks of L-ethyl lactate.
[0210] Table 1 ...
Embodiment 2
[0212] Example 2 The preparation method of L-ethyl lactate solvate crystal form A
[0213] Weigh 1.23g of Acalabrutinib free base sample into a 20mL glass bottle, add 12.0mL L-ethyl lactate / ethyl acetate (1:1, v / v). After magnetic stirring at 50° C. for 3 days, 1.34 g of solid was obtained by separation and drying. After detection, the obtained solid is the crystal form A of the present invention, and its XRPD data is shown in Table 2. The crystal form A obtained in this example has the same or similar XRPD pattern as the crystal form A in Example 1, is the same crystal form, and has the same properties.
[0214] Table 2
[0215]
[0216]
Embodiment 3
[0217] Example 3 Purification of L-ethyl lactate solvate crystal form A
[0218] The purity of the free base solid and the L-lactate ethyl lactate solvate crystal form A of the present invention was measured by HPLC, and the purity change was calculated.
[0219] The HPLC purity test results show that the L-lactate ethyl lactate solvate crystal form A of the present invention has a remarkable purification effect. The purity of the free base solid is 98.93%, and the purity of the L-lactate ethyl lactate solvate crystal form A of the present invention is 99.63%, with a purity increase of 0.70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com