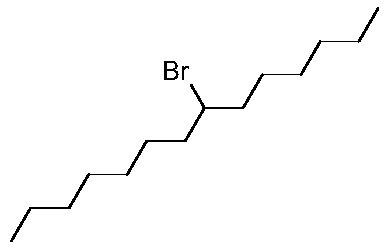
Synthesis method of 7-bromotetradecane
A technology of bromotetradecane and a synthesis method, applied in the field of material synthesis, can solve the problems of difficulty in obtaining 7-bromotetradecane, inability to separate 6-bromotetradecane, and difficulty in obtaining raw materials. The effect of high product purity and mild reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
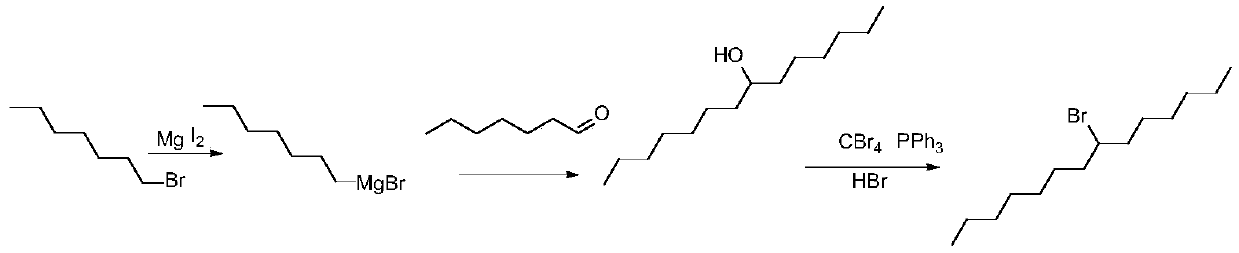
[0031] The present invention provides a kind of synthetic method of 7-bromotetradecane, and its synthetic route and synthetic steps are as follows:
[0032]
[0033] Step 1, synthesis of Grignard reagents:
[0034] Magnesium is added to the reaction flask, 1-bromoheptane and tetrahydrofuran or 2-methyltetrahydrofuran are prepared into a solution, and added dropwise to the reaction flask for reaction to generate Grignard reagent;
[0035] Step 2, synthesis of 7-tetradecyl alcohol:
[0036] Add heptanal dropwise to the system after the reaction in step 1, keep the reaction for 30-60 minutes, quench the reaction with dilute hydrochloric acid, add acetic acid for extraction; then combine the organic phases, then wash the organic phases with saturated saline, and wash them with anhydrous Dry over sodium sulfate, filter, and desolventize to obtain the crude product of 7-tetradecyl alcohol;
[0037] Step three, synthesis of 7-bromotetradecane:
[0038] Dissolve the 7-tetradecyl...
Embodiment 1
[0050] The present embodiment provides a kind of synthetic method of preferred 7-bromotetradecane, comprises the following steps:
[0051] Step 1: Magnesium (2.88g, 0.12mol) is added to the reaction flask, 1-bromoheptane (17.8g, 0.1mol) and tetrahydrofuran (150ml) are prepared into a solution, which is added dropwise to the reaction flask to start the dropwise addition at room temperature After initiation, the temperature of the reaction system was lowered to 0°C, and the remaining tetrahydrofuran solution of 1-bromoheptane was continuously added dropwise, and the temperature was controlled at 0°C. After the addition was completed, the Grignard reagent was obtained by keeping the temperature at 0°C for 1 hour;
[0052] Step 2: Add heptanal (11.4g, 0.1mol) dropwise to the reaction system in Step 1, and keep the temperature below 5°C. After the addition, keep warm at 0°C for 60min, and quench with 2N dilute hydrochloric acid (150ml). The reaction was quenched, and acetic acid (3...
Embodiment 2
[0057] The present embodiment provides a kind of synthetic method of preferred 7-bromotetradecane, comprises the following steps:
[0058] Step 1, magnesium (2.88g, 0.12mol) was added to the reaction flask, 1-bromoheptane (17.8g, 0.1mol) and 2-methyltetrahydrofuran (150ml) were prepared into a solution, and added dropwise to the reaction flask Start dropwise addition at room temperature until initiation, then cool down the reaction system to 0°C, continue to dropwise add the remaining 1-bromoheptane in 2-methyltetrahydrofuran solution, control the temperature at 0°C, after the dropwise addition, keep the reaction at 0°C for 1 hour Get the Grignard reagent;
[0059]Step 2: Add heptanal (11.4g, 0.1mol) dropwise to the reaction system in Step 1, and keep the temperature below 5°C. After the addition, keep the reaction at 10°C for 40 minutes, and quench with 2N dilute hydrochloric acid (150ml). To quench the reaction, add acetic acid (3*150ml) to extract three times, combine the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com