A long-lived plasma cell that secretes PD-1 antibody and its preparation method and application
A PD-1, plasma cell technology, applied in the field of biomedicine, can solve the problem of low efficiency of large fragment exogenous gene knock-in, and achieve the effect of improving the infection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Preparation of long-lived plasma cells secreting PD-1 antibody
[0058] 1. Plasmid construction and B lymphocyte culture
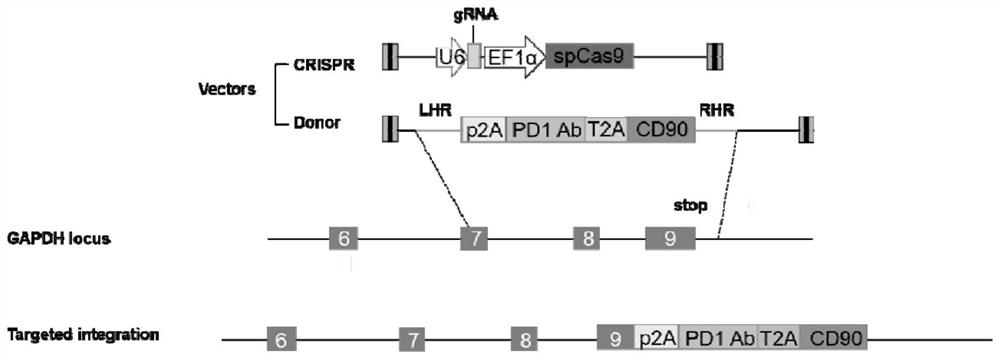
[0059] (1) Construction of Cas9 / sgRNA expression lentiviral plasmid
[0060] Design sgRNA with Zhang Feng's laboratory sgRNA design tool ((https: / / zlab.bio / guide-design-resources), synthesize oligonucleotide primers, form oligonucleotide primer pairs through annealing reaction, and clone into BsmBI digestion The lentiCRISPR v2 (Addgene) plasmid.
[0061] Annealing reaction system:
[0062] Oligo 1 (100μM) 1μl Oligo 2 (100μM) 1μl 10×NEB buffer 2 5μl ddH 2 O
Make up to 50μl
[0063] Annealing reaction procedure: heating at 95°C for 5 minutes, heating at 72°C for 10 minutes, and cooling to room temperature naturally.
[0064] (2) Construction of homologous recombination template lentiviral plasmid
[0065] Human peripheral blood lymphocytes (PBMCs) were isolated, and genomic DNA was extracted with...
Embodiment 2
[0094] Example 2 sgRNA sequence screening
[0095] In order to determine the appropriate sgRNA sequence, three sgRNAs were designed near the terminator of the GAPDH 3'-UTR coding region, as shown in Table 1, and cloned into the lentiCRISPR v2 vector respectively. Construction of viral plasmids. Then HEK293T cells were transfected, and the genomic DNA was extracted after puromycin selection.
[0096] Table 1 Sequences of sgRNAs
[0097]
[0098] Detection of mutants by T7E1 digestion:
[0099] a. Collect cells to extract genomic DNA, use this as a template, and use PCR to amplify DNA fragments with mutation points (sgRNA target sequences);
[0100] b. Configure the following reaction system:
[0101] PCR product 300ng 10×NEB buffer 2 2μl ddH 2 O
Make up to 20μl
[0102] Heating at 95°C for 5 minutes, naturally cooling to room temperature, and performing annealing reaction;
[0103] c. Add 1 μl T7E1 enzyme for digestion, and incubate at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com