Neostigmine mesylate injection and preparation method thereof
A technology of neostigmine methosulfate and injection, which is applied in the field of medicine, can solve problems such as microbial contamination, and achieve the effects of low impurity content, high sterility assurance level, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0035] The preparation of embodiment 1~4 neostigmine methylsulfate injection
[0036] 1, the proportioning ratio of each component of embodiment 1~4 is as shown in table 2:
[0037] Table 2
[0038]
[0039] 2. Preparation method:
[0040] Take neostigmine methosulfate and isotonic regulator, add water for injection to dissolve, then add pH regulator to adjust the pH of the product, filter through 0.45 μm and 0.22 μm PVDF microporous membranes successively, potting and sterilizing, namely Obtain described neostigmine methylsulfate injection.
[0041] 3. Sample quality test results
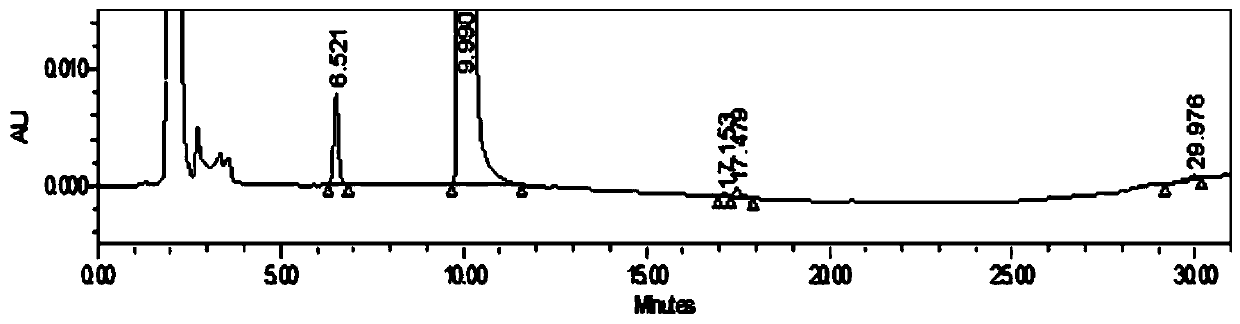
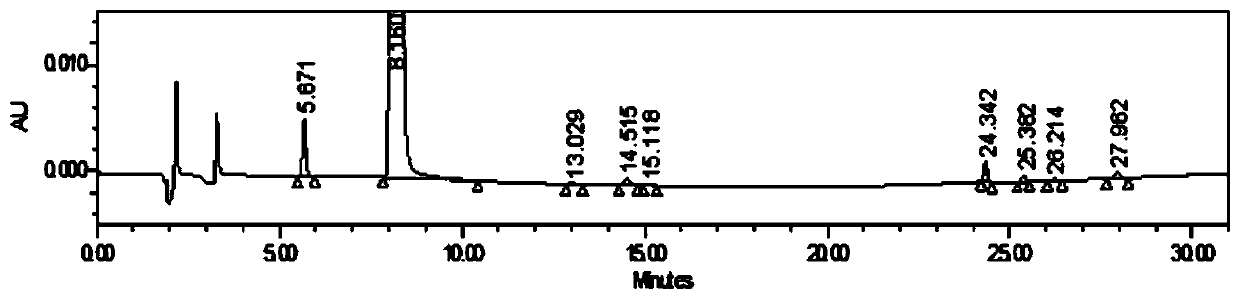
[0042] Figure 1-3 Be respectively the neostigmine methylsulfate injection prepared by embodiment 1, the neostigmine methylsulfate injection of the commercially available product 2 French MedaPharma company and the neostigmine methylsulfate injection produced and listed by a domestic certain manufacturer of the commercially available product 3 Impurity chromatogram.
[0043] The neostigmin...
Embodiment 6
[0049] Embodiment 6 product stability research
[0050] 1. The neostigmine methosulfate injection prepared in Example 5 was accelerated at 40°C for 6 months, and the stability of the product was tested, as shown in Table 4:
[0051] Table 4
[0052]
[0053] As can be seen from the data in the table, the neostigmine methylsulfate injection prepared by the present invention is accelerated at 40 DEG C for 6 months, has higher stability, and all impurities (especially impurity I) have no obvious increase , but also to see new impurities.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com