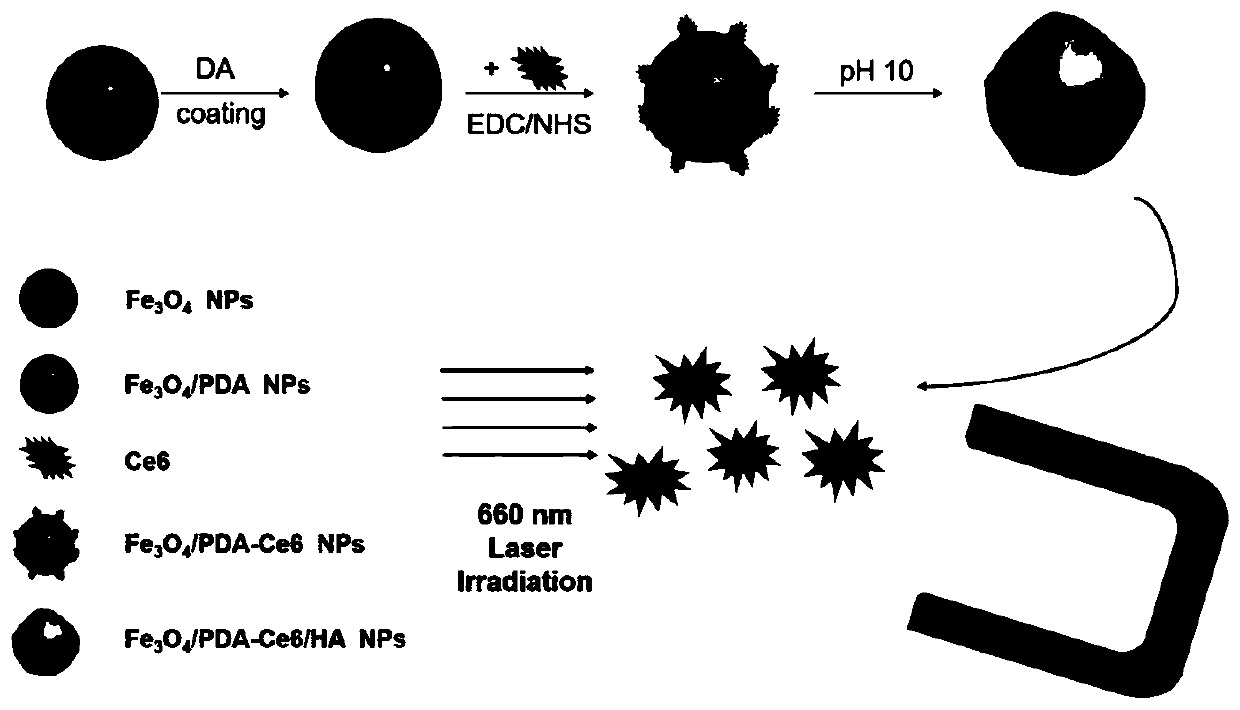
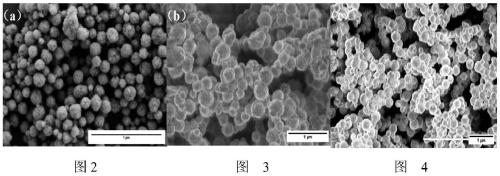
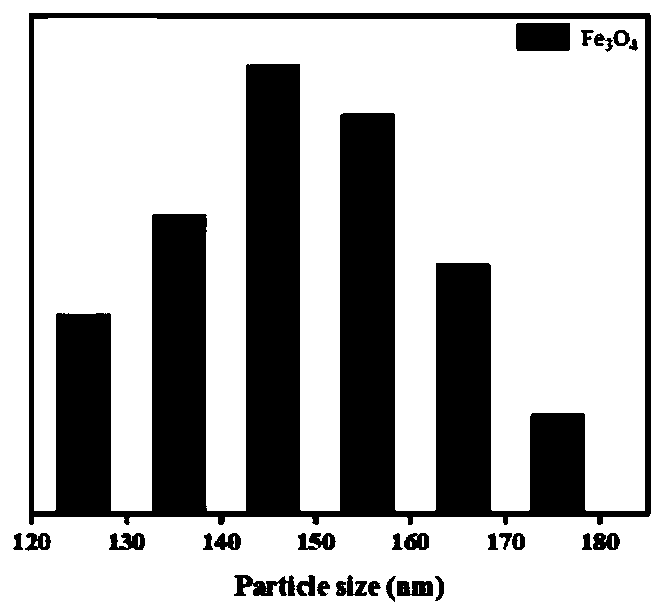
Hydroxyapatite-coated magnetic drug-loaded nanoparticles, preparation method thereof and application of hydroxyapatite-coated magnetic drug-loaded nanoparticles in preparation of osteosarcoma phototherapy drugs
A technology of magnetic nanoparticles and hydroxyapatite, which is applied in the fields of pharmaceutical formulation, drug combination, drug delivery, etc., can solve problems such as poor targeting effect, ineffective promotion of osteoblast differentiation, phototoxicity and side effects of normal tissues, etc. , to achieve the effects of easy operation, promotion of osteoblast differentiation, and accurate drug targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] 1. Preparation of hydroxyapatite-coated magnetic drug-loaded nanoparticles
[0073] The preparation method of hydroxyapatite-coated magnetic drug-loaded nanoparticles in this embodiment comprises the following steps:
[0074] (1) preparing ferroferromagnetic nanoparticles;
[0075] Specifically include: take 2g FeCl 3 ·6H 2 O, 3g of anhydrous sodium acetate and 1mL PEG-200 were stirred and dissolved in 36mL of ethylene glycol at room temperature, and the rotation speed was 600rpm to obtain a mixed solution; the mixed solution was poured into a 50mL reactor, and hydrothermally reacted at 200°C for 4h, and then Cool to room temperature, wash with magnet centrifuge and vacuum dry to obtain Fe 3 o 4 magnetic nanoparticles.
[0076] (2) In a slightly alkaline environment, dopamine hydrochloride is added to the ferroferric oxide magnetic nanoparticle suspension to react to obtain the polydopamine magnetic nanoparticle core;
[0077] Specifically include: take 1g Fe 3 o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com