Broad-spectrum bactericidal low-toxicity low-residue growth-promoting prothioconazole manganese-zinc compound and composition thereof
A technology of prothioconazole manganese zinc and prothioconazole, applied in the field of pesticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1A
[0113] Embodiment 1-1A prothioconazole mancozeb [(C 14 h 14 Cl 2 N 3 OS) 2 (Mn) 0.5 (Zn) 0.5 ] preparation
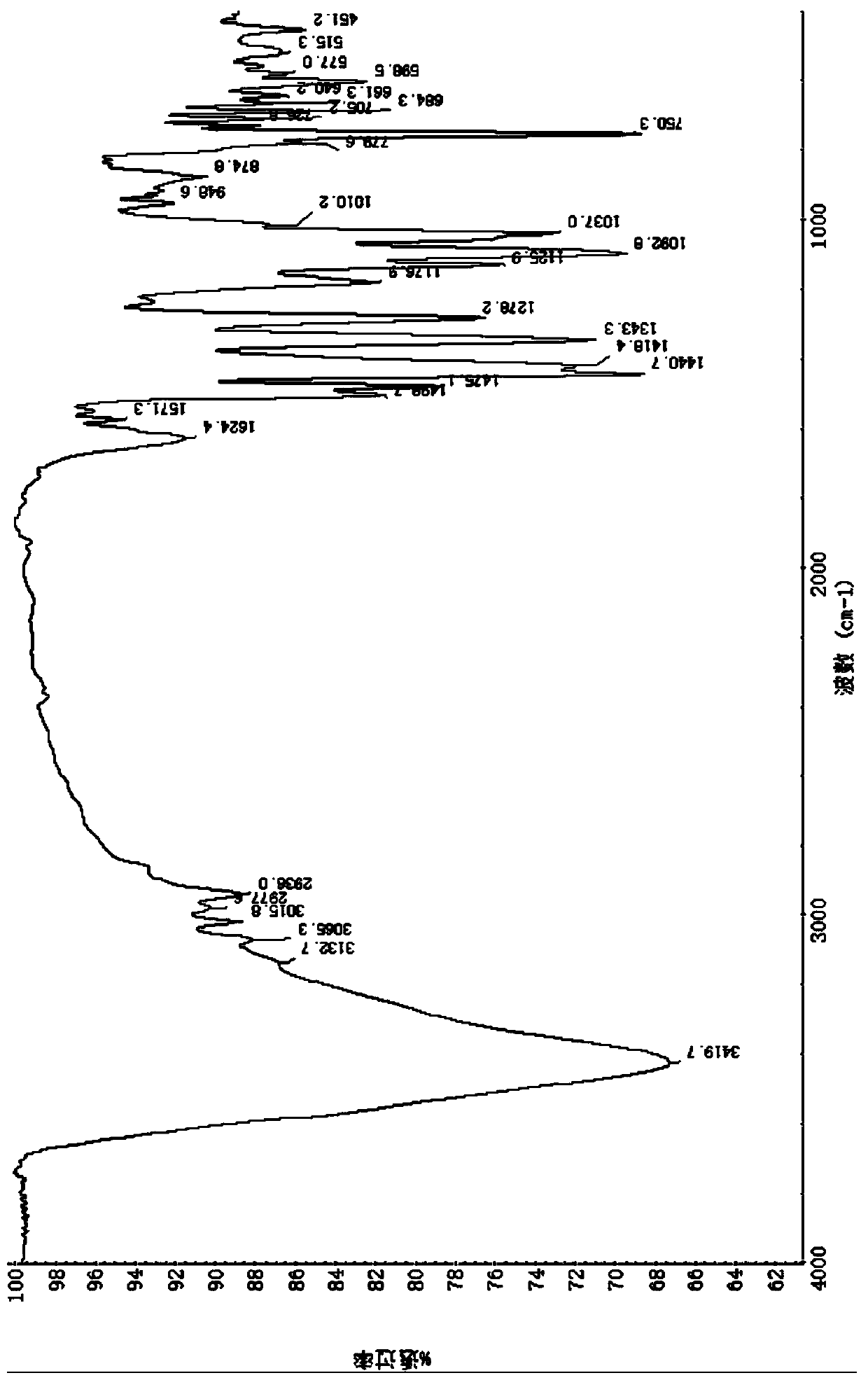
[0114] At room temperature, add 3.443g of prothioconazole (0.01mol) to a 250ml flask, add 20ml of water, stir, add an appropriate amount of 5% aqueous sodium hydroxide solution, stir until just completely dissolved, then add manganese sulfate monohydrate (0.005mol) 0.845g and 1.438g of zinc sulfate heptahydrate (0.005mol) dissolved in an appropriate amount of water, add 10ml of ethanol, stir at about 40°C for half an hour, take it out and let it cool, filter with suction, wash the solid with a small amount of water until there is no sulfate ion, pump Filtration, the obtained solid was diluted and dried in vacuum at about 80°C for about 5 hours to obtain 3.1 g of brownish gray solid; melting point: discoloration at about 142°C; theoretical value of elemental analysis: C45.04%, H 3.78%, N11.26%, Cl 18.99% , S 8.59%; measured value: C44.96%, H 3.83%, N 11.21%, Cl 18...
Embodiment 1-2A
[0115] Embodiment 1-2A prothioconazole mancozeb [(C 14 h 14 Cl 2 N 3 OS) 2 (Mn) 0.5 (Zn) 0.5 ] Preparation of 2 hydrate
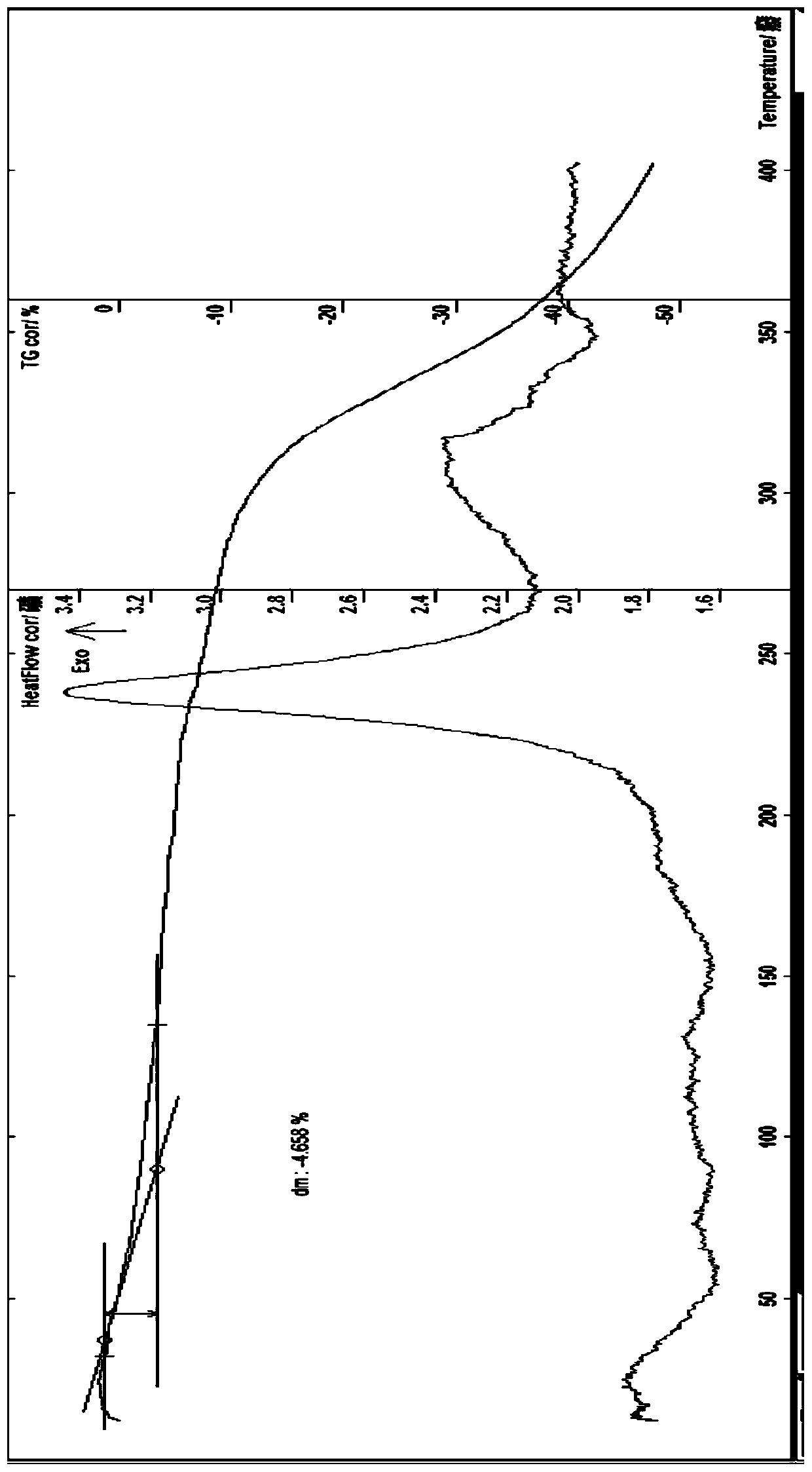
[0116] At room temperature, add 6.886g of prothioconazole (0.02mol) to a 250ml flask, add 30ml of water, stir, add an appropriate amount of 5% aqueous sodium hydroxide solution, stir until just completely dissolved, then add manganese sulfate monohydrate (0.01mol) 1.69g and 2.876g of zinc sulfate heptahydrate (0.01mol) dissolved in an appropriate amount of water, add 10ml of ethanol, stir at about 35°C for 0.5h, take it out and let it cool, filter with suction, wash the solid with a small amount of water until there is no sulfate ion, filter with suction , the obtained solid was diluted and air-dried at about 50°C for about 4 hours to obtain 6.0g of brownish gray solid; melting point: discoloration at about 141°C; moisture measured by Karl Fischer method was 4.57%; thermal analysis: weight loss on the platform was about 4.66% (see attached figure 1 )...
Embodiment 1-3A
[0117] Embodiment 1-3A prothioconazole mancozeb [(C 14 h 14 Cl 2 N 3 OS) 2 (Mn) 0.8 (Zn) 0.2 ] preparation
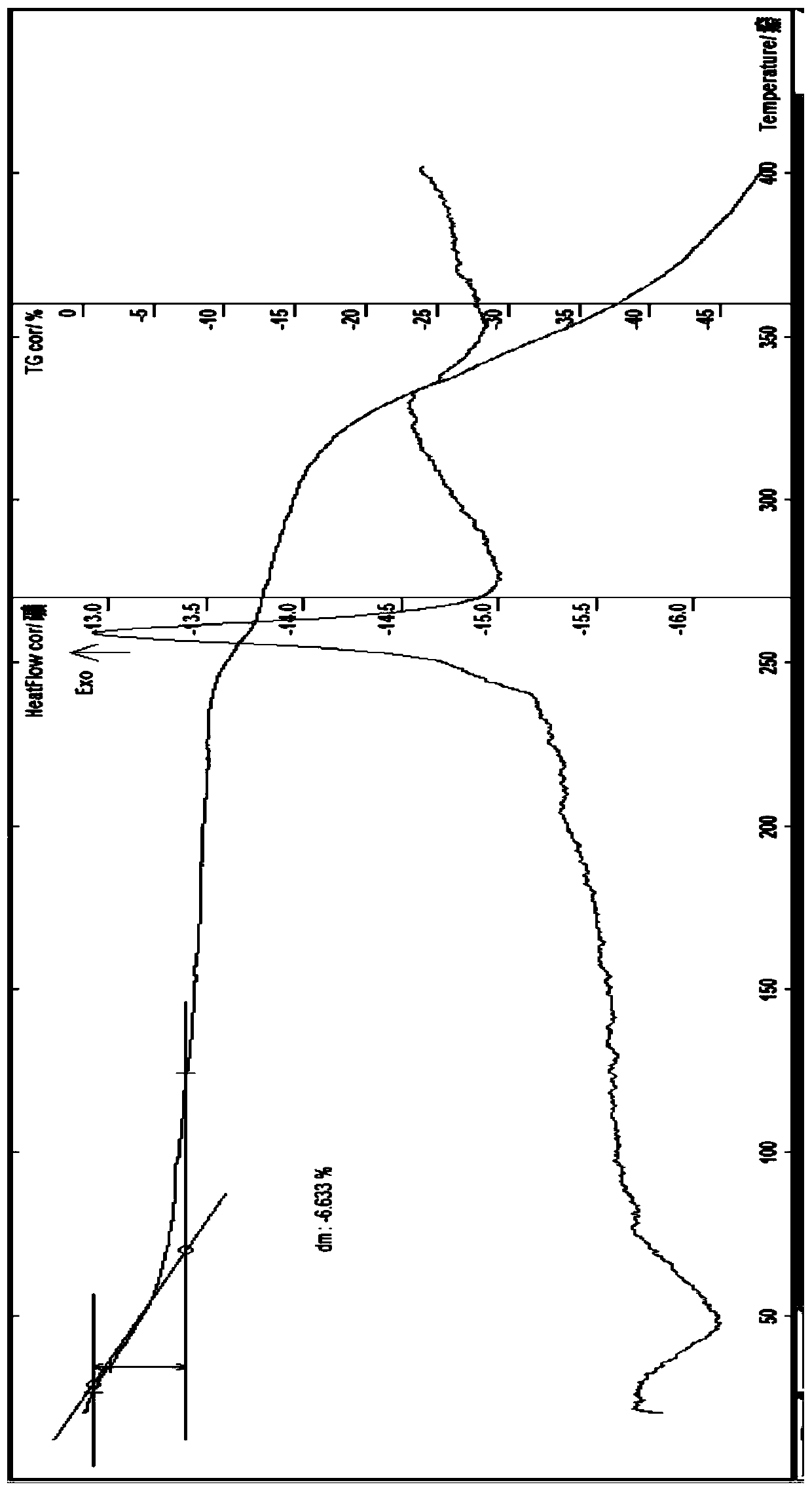
[0118] At room temperature, add prothioconazole (0.02mol) 6.886g in a 250ml flask, add water 30ml, stir, add 5% sodium hydroxide solution, stir to make the solid just dissolve, then add manganese sulfate monohydrate (0.008mol) 1.352 g and 0.576g of zinc sulfate heptahydrate (0.002mol) dissolved in an appropriate amount of water, add 10ml of ethanol, stir at about 40°C for 0.5h, take it out and let it cool, filter with suction, wash the solid with a small amount of water and ethanol until there is no sulfate ion, Suction filtration, the obtained solid was diluted and dried in vacuum at about 85°C for about 4 hours to obtain 5.2g of brownish gray solid; melting point: discoloration at about 150°C; theoretical value of elemental analysis: C45.23%, H 3.80%, N11.30%, Cl 19.07 %, S 8.63%; measured value: C45.31%, H 3.87%, N 11.24%, Cl 18.99%, S8.75%; content analysis (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com