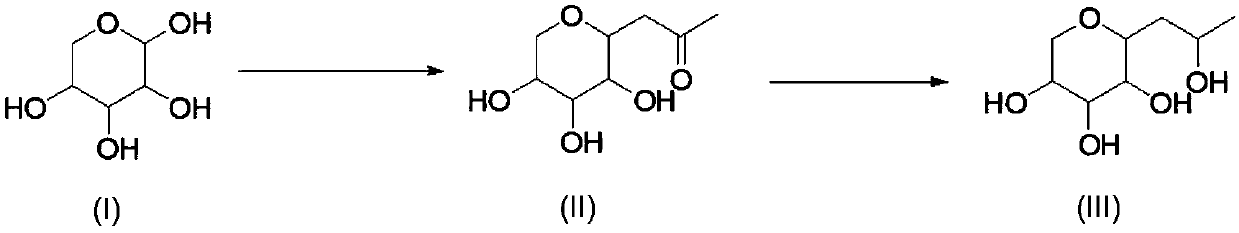
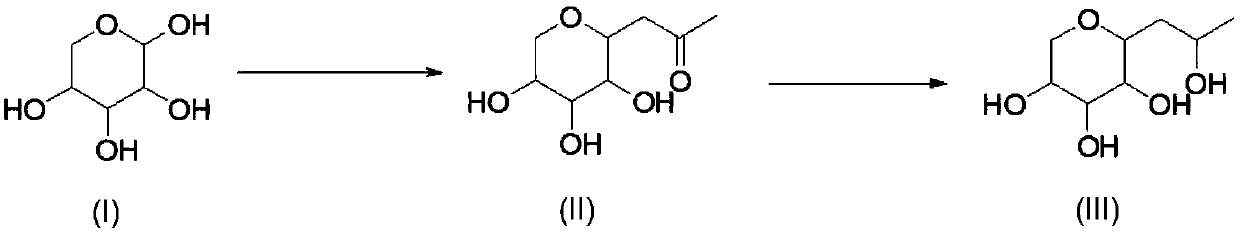
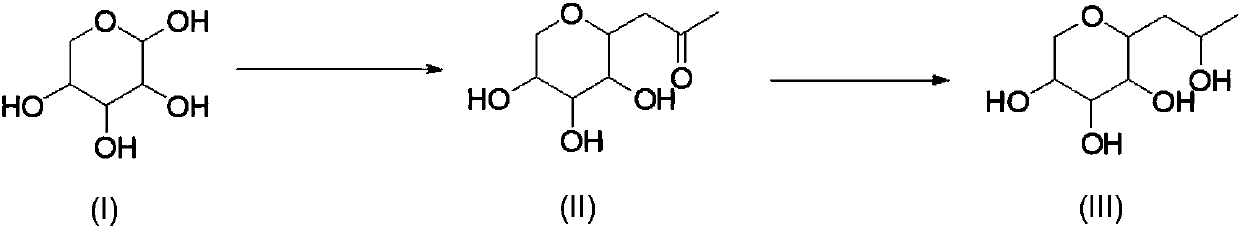
Synthetic method of hydroxypropyl tetrahydropyrantriol
A technology of hydroxypropyltetrahydropyrantriol and synthesis method, applied in the field of fine chemicals, can solve problems such as insufficient preparation yield, achieve the effects of high product purity, avoid acid residues, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Add 1.5kg of xylose, 1kg of sodium carbonate, 1.4kg of acetylacetone, 15L of water into the reaction bottle, and react at 80-85°C for 20h. After the reaction is completed, return to room temperature, extract with ethyl acetate (3Lx3), and use saturated sodium bisulfate solution Adjust the pH to 4-5, spin the water to obtain a viscous residue, add 10L of ethanol, filter with suction, wash the filter cake with 2L of ethanol, and the obtained mother liquor is directly used for feeding in the next step.
[0032] 1 HNMR (D2O, 400MHz): δ3.79-3.81(m, 1H), 3.62-3.63(m, 1H), 3.43-3.45(m, 1H), 3.31-3.34(m, 1H), 3.19-3.21(m , 1H), 3.11-3.13(t, 1H), 2.92-2.93(m, 1H), 2.58-2.62(m, 1H), 2.25(s, 3H).
[0033]
[0034] Pump the solution obtained in the previous step into the reaction bottle, add 430g of sodium borohydride in batches at 0-5°C, control the reaction temperature not to exceed 40°C during the addition, after the addition is complete, keep the reaction at roo...
Embodiment 2
[0037] Add 1.5kg of xylose, 0.8kg of sodium bicarbonate, 1.4kg of acetylacetone, 15L of water, and react at 95-100°C for 6h in the reaction flask. After the reaction is complete, return to room temperature, extract with ethyl acetate (3L×3), and The sodium bisulfate solution was adjusted to pH=4-5, and the water was spin-dried to obtain a viscous residue. Add 10 L of ethanol, filter with suction, wash the filter cake with 2 L of ethanol, and the obtained mother liquor was directly used for feeding in the next step.
[0038] Pump the solution obtained in the previous step into the reaction bottle, add 350 g of sodium cyanoborohydride in batches at 10-15 ° C, control the reaction temperature during the addition process to not exceed 40 ° C, after the addition is complete, keep the reaction at room temperature for 3 hours, add saturated Sodium bisulfate solution adjusted the pH to 5-6, a large amount of solids precipitated, suction filtered, the solids were washed with 5 L of abso...
Embodiment 3
[0040] Add 1.5kg of xylose, 1kg of sodium carbonate, 1.4kg of acetylacetone, 15L of water, and react at 90-95°C for 10h in the reaction flask. After the reaction is completed, return to room temperature, extract with ethyl acetate (3L×3), and use The sodium solution was adjusted to pH = 4-5, and the water was spin-dried to obtain a viscous residue. Add 10 L of ethanol, filter with suction, wash the filter cake with 2 L of ethanol, and the obtained mother liquor was directly used for feeding in the next step.
[0041] Pump the solution obtained in the previous step into the reaction bottle, add 350 g of sodium triacetylborohydride in batches at 30-40 ° C, control the reaction temperature during the addition process to not exceed 40 ° C, after the addition is completed, keep the reaction at room temperature for 3 hours, add saturated The sodium bisulfate solution was used to adjust the pH to 5-6, a large amount of solids were precipitated, filtered by suction, the solids were was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com