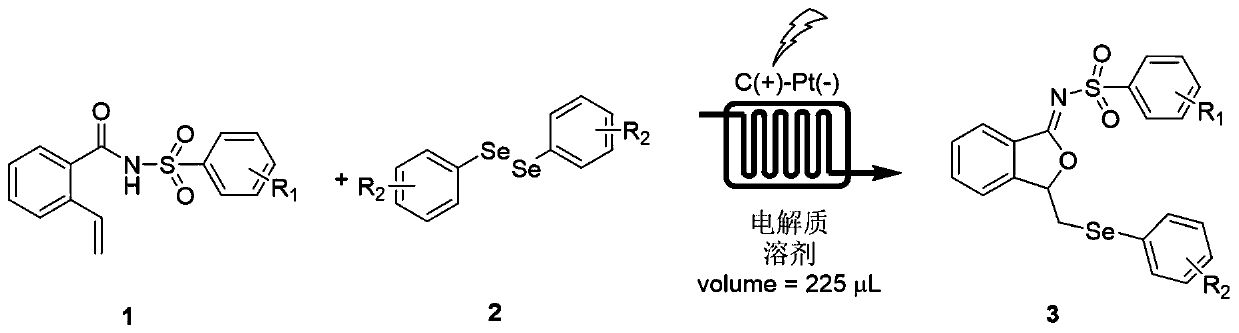
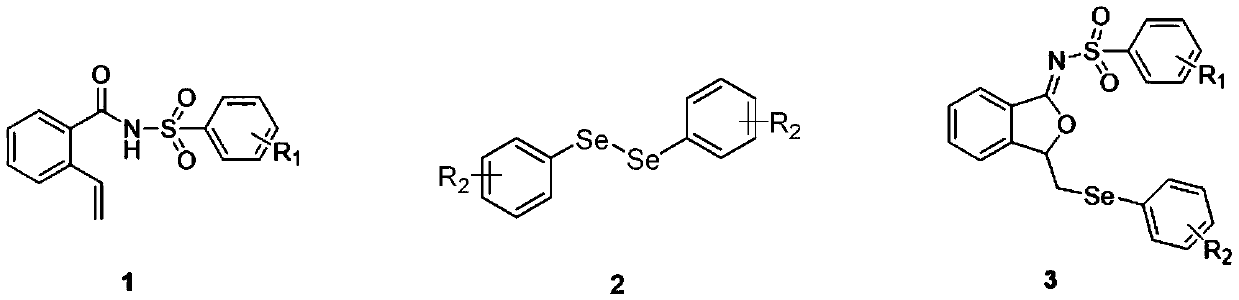
Method for continuously preparing isobenzofuran compounds by using micro-channel reaction device
A microchannel reaction and compound technology, applied in organic chemistry, electrodes, electrolysis processes, etc., can solve problems such as the need for expensive catalysts and long reaction process cycles, and achieve the effects of good industrial amplification potential, low cost, and easy preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthesis of embodiment 1 compound 3a:
[0046] 0.2 mmol (0.060 g) of compound 1a, 0.2 mmol (0.062 g) of diphenyldiselenide 2a and 0.4 mmol of lithium perchlorate (0.043 g) were dissolved in acetonitrile (8 mL) to obtain a homogeneous solution A, which was added to In the syringe pump a; the injection flow rate of the syringe pump a is 450 μL / min; the applied current is 15 mA; the microchannel reactor reaction volume V=225 μL, and the reaction time is 30 s; The yield of the product calculated by HPLC was 86%, and the product 3a was obtained after separation by column chromatography. 1 H NMR (400MHz, Chloroform-d) δ7.92 (d, J = 8.2Hz, 2H), 7.82 (d, J = 5.6Hz, 1H), 7.40 (q, J = 7.8, 5.7Hz, 2H), 7.36 –7.31(m,1H),7.28(d,J=7.2Hz,2H),7.22(d,J=8.1Hz,2H),7.20–7.15(m,1H),7.12(t,J=7.2Hz, 2H), 5.83–5.77(m, 1H), 3.31(dd, J=13.3, 4.6Hz, 1H), 3.13(dd, J=13.3, 6.6Hz, 1H), 2.34(s, 3H). 13C NMR (101MHz, Chloroform-d) δ166.04, 145.43, 142.46, 137.18, 133.17, 132.78, 128.79, 128.26...
Embodiment 2
[0047] The synthesis of embodiment 2 compound 3a:
[0048] 0.2 mmol (0.060 g) of compound 1a, 0.2 mmol (0.062 g) of diphenyldiselenide 2a and 0.4 mmol of tetrabutylammonium hexafluorophosphate (0.155 g) were dissolved in acetonitrile (8 mL) to obtain a homogeneous solution A , added to syringe pump a; the injection flow rate of syringe pump a is 450μL / min; the applied current is 15mA; the microchannel reactor reaction volume V=225μL, and the reaction time is 30s; In the reaction liquid, the yield of the product calculated by HPLC was 70%, and the product 3a was obtained after separation by column chromatography.
Embodiment 3
[0049] The synthesis of embodiment 3 compound 3a:
[0050] 0.2 mmol (0.060 g) of compound 1a, 0.2 mmol (0.062 g) of diphenyldiselenide 2a and 0.4 mmol of tetrabutylammonium tetrafluoroborate (0.132 g) were dissolved in acetonitrile (8 mL) to obtain a homogeneous solution A , added to syringe pump a; the injection flow rate of syringe pump a is 450μL / min; the applied current is 15mA; the microchannel reactor reaction volume V=225μL, and the reaction time is 30s; In the reaction liquid, the yield of the product calculated by HPLC was 77%, and the product 3a was obtained after separation by column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com