Application of hydroxyoctadecadienoic acid in preparation of alpha-glucosidase inhibitor drugs and separation method of alpha-glucosidase inhibitor drugs
A technology of octadecadienoic acid and glucosidase, which is applied in the field of hypoglycemic drugs, can solve the problems of easy loss of active compounds, heavy workload, and tediousness, and achieve good application prospects and the effect of lowering blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
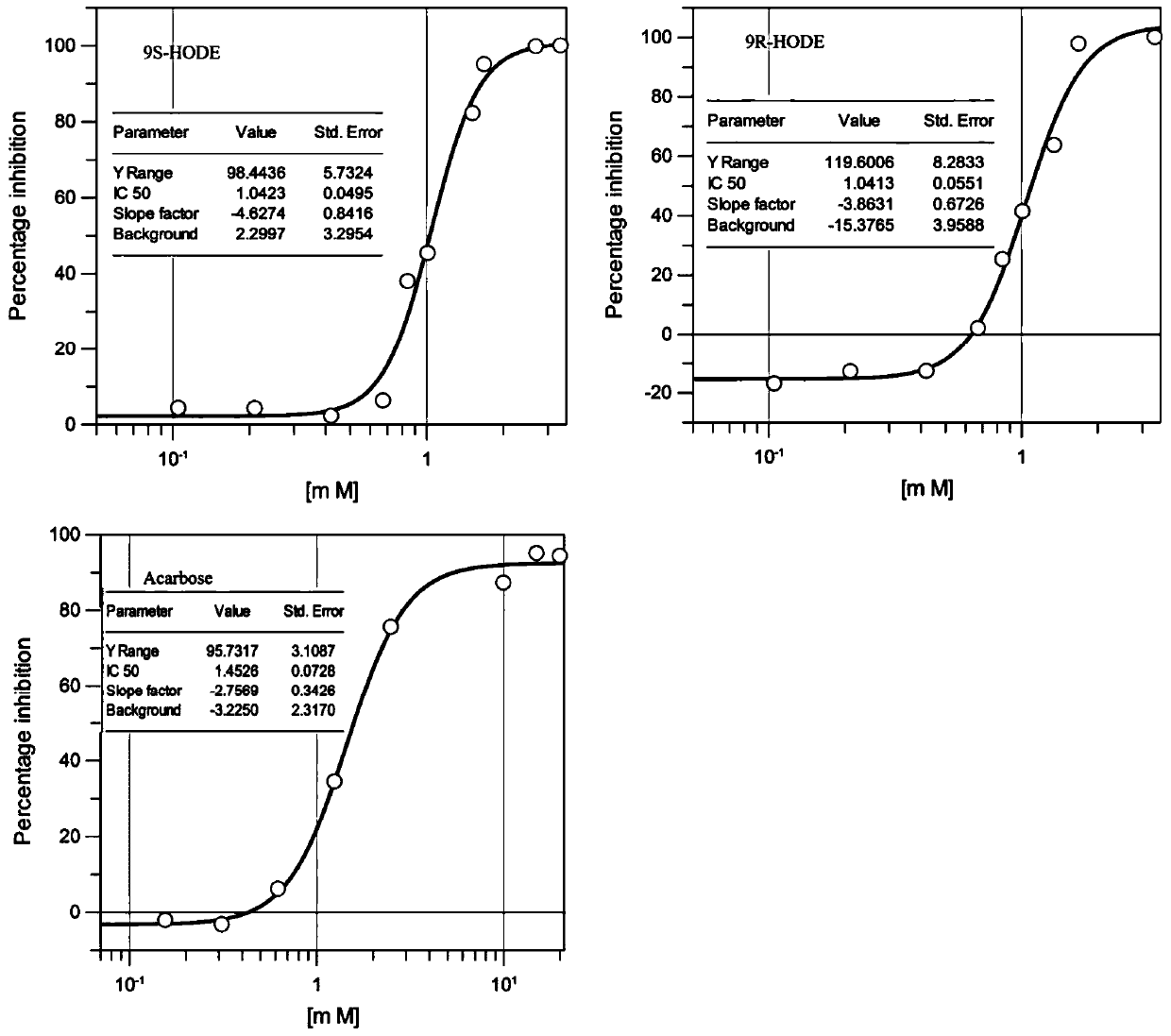
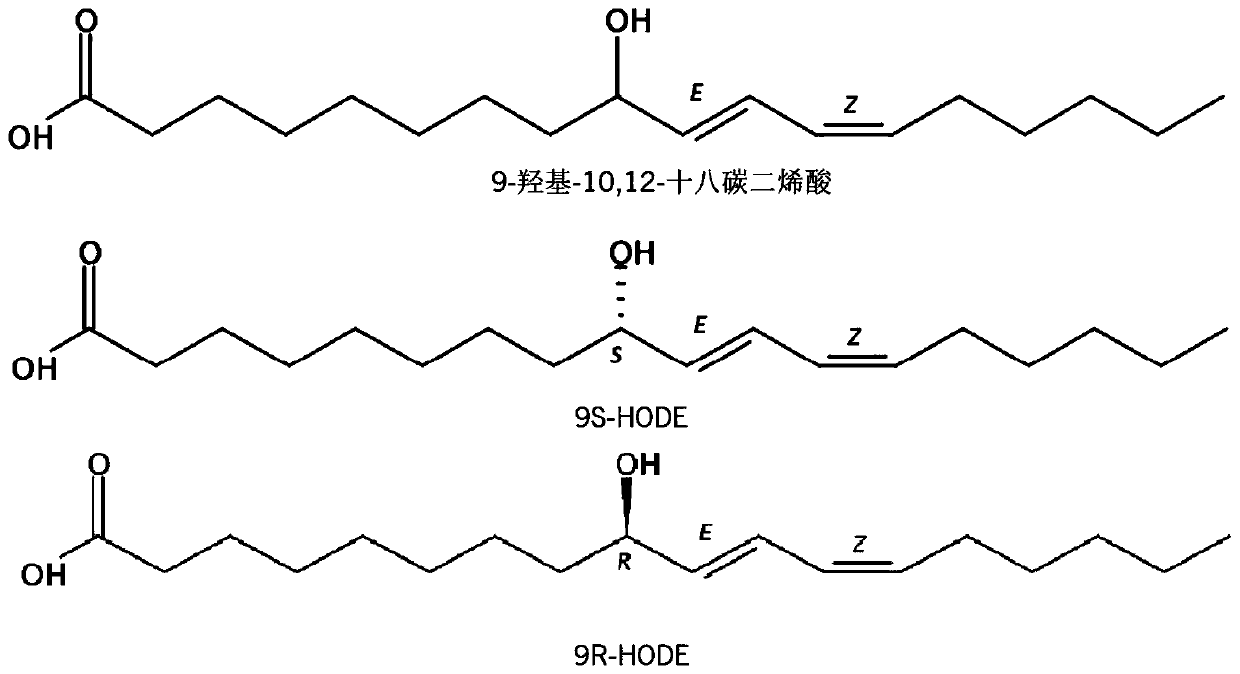
[0035] Example 1 Identification of α-glucosidase inhibitory activity of 9-hydroxyl-10,12-octadecadienoic acid
[0036] Buy two standard products of 9-hydroxy-10,12-octadecadienoic acid: (9S,10E,12Z)-9-hydroxy-10,12-octadecadienoic acid (9S-HODE for short) and (9R,10E,12Z)-9-hydroxyl-10,12-octadecadienoic acid (abbreviated as 9R-HODE) (Cayman Chemical Company, MI, USA), was determined for its α-glucosidase inhibitory activity, expressed as A Carbose was used as a positive control.
[0037] The determination method is as follows:
[0038] The sample was dissolved in DMSO, prepared into a 40mg / mL solution, diluted with DMSO into different concentration gradients, 10 μL each was placed in a 96-well plate, and 90 μL of 0.1M phosphate buffer (pH7.5, 0.02% NaN 3 ), add 80 μL of α-glucosidase dissolved in the above-mentioned phosphate buffer solution with a concentration of 0.05 U / mL, mix the mixture thoroughly and place it in an incubator at 28°C for 10 min, and then add 20 μL of p...
Embodiment 2
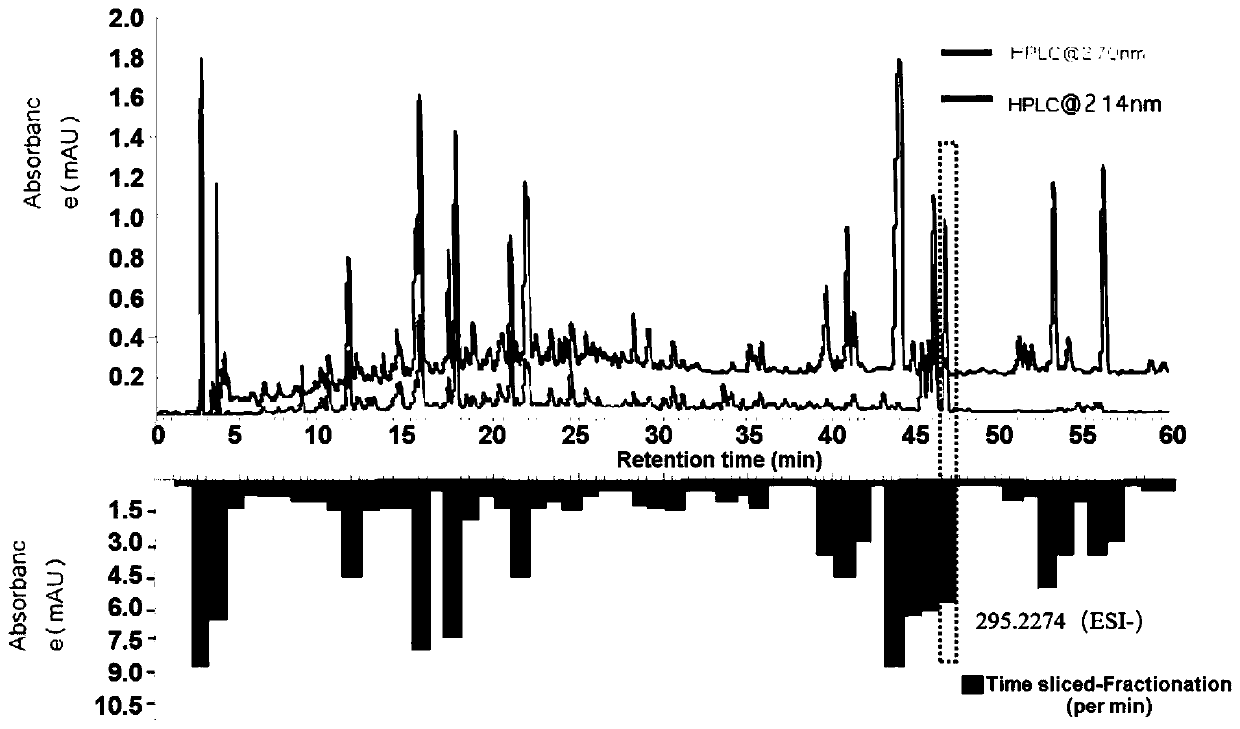
[0040] Example 2 Separation of 9-antelope-10,12-octadecadienoic acid in Digupi
[0041] The purpose of this example is to separate 9-hydroxy-10,12-octadecadienoic acid from Cortex Dictica.
[0042] The separation steps are as follows:
[0043] 1. Rough extraction
[0044] Crush Digupi, weigh 10 g of the sample into a stoppered Erlenmeyer flask, add 100 mL of ethyl acetate, shake for 1 hour, place it for 48 hours, shake for 10 minutes, filter, and dry the filtrate in a vacuum dryer to obtain a crude extract.
[0045] 2. Separation
[0046] The crude extract was dissolved in methanol to prepare a 40 mg / mL solution, filtered through a 0.45 μm pore-diameter filter membrane, and analyzed by liquid chromatography, and a fraction was collected every 1 min.
[0047] The chromatographic conditions are: the mobile phase flow rate is 0.5mL / min, and the mobile phase consists of (A) 5% acetonitrile dissolved in water (comprising 0.1% formic acid) and (B) 95% acetonitrile dissolved in wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com