Application of POLR2A inhibitor in preparation of medicine
A technology for preparing drugs and inhibitors, which is applied in the field of biomedicine, and can solve problems such as the incomplete understanding of the pathological mechanism of estrogen and the increased risk of cardiovascular events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] In vitro experiments demonstrate that POLR2A promotes osteoclast differentiation
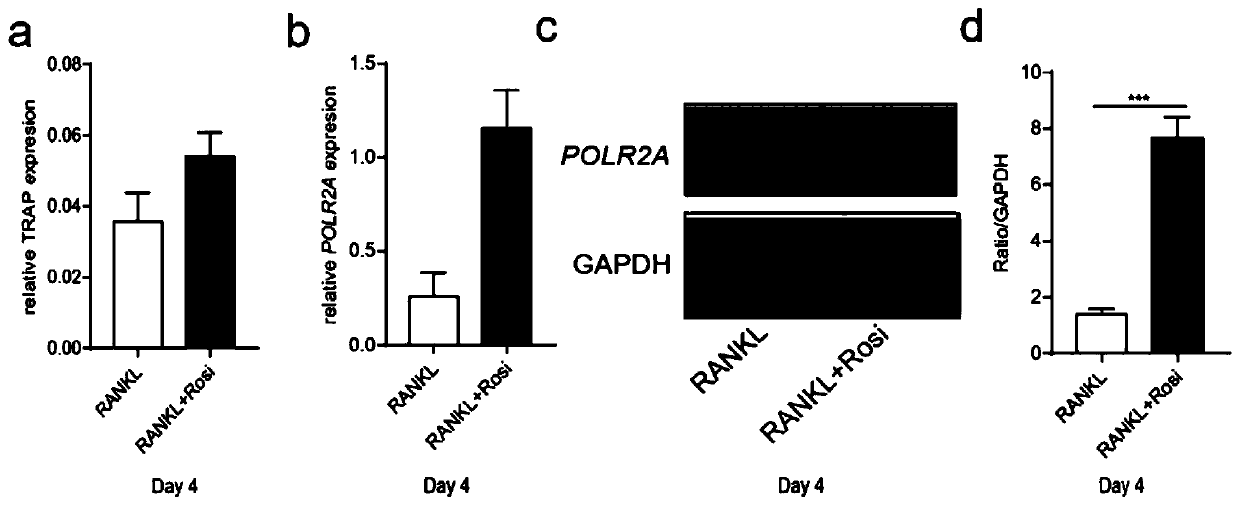
[0095] The inventors of the present application first assessed the expression of POLR2A in bone marrow osteoclasts. After 4 days of culture, RANKL increased the expression of osteoclast marker tartrate-resistant acid phosphatase (TRAP), and the expression of TRAP was further increased after adding the osteoporosis-inducing drug rosiglitazone ( figure 1 Middle a). The expression of POLR2A increased in the RANKL group, and further increased under the stimulation of rosiglitazone ( figure 1 middle b~d).
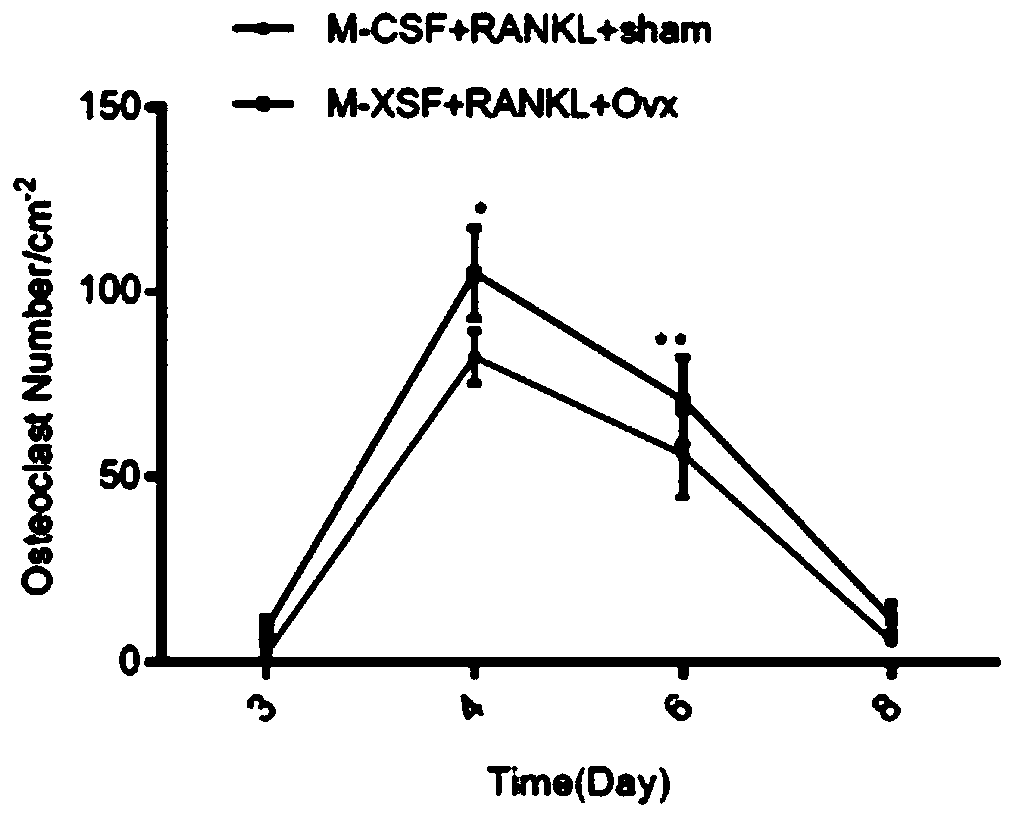
[0096] The inventors of the present application then studied the growth cycle of osteoclasts ( figure 2 ). On the 3rd day from the start of culture, after staining with TRAP, almost no osteoclasts were detected in the RANKL and rosiglitazone groups. On day 4, the inventors of the present application detected the maximum number of osteoclasts in both groups. As the incubation time inc...
Embodiment 2
[0099] Bone is protected against Ovx-induced osteoporosis with α-Amanitin
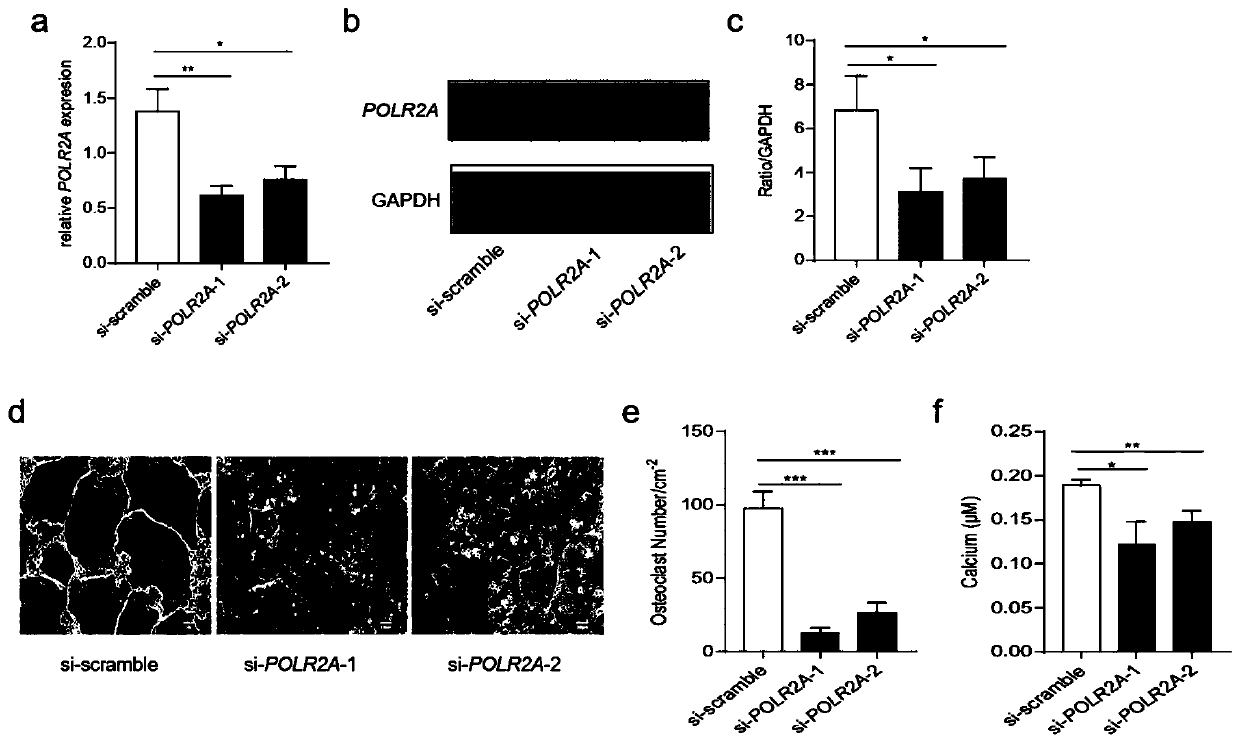
[0100] The inventors of the present application demonstrated that the overexpression of POLR2A can promote bone loss in previous studies, so the inventors of the present application evaluated the curative effect after treatment with α-Amanitin, a specific inhibitor of POLR2A. It has been reported that α-Amanitin specifically inhibits POLR2A (Bensaude et al. 2011; Lindell et al., 1970), so the inventors of the present application evaluated the effect of α-Amanitin on osteoclastogenesis. It is reported that α-Amanitin has cytotoxicity (Letschert et al. 2006), so the inventors of the present application first tested the cytotoxicity of α-Amanitin at different concentrations. MTT results showed that there was no significant difference in cell viability between the control group and the α-Amanitin group (from 0 to 80ng / ml group ( Figure 5 middle a~b). Bone marrow osteoclast production assay showed that α...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com