Preparation method of 2, 5-furandimethanol
A technology of furandimethanol and hydroxymethylfurfural, which is applied in chemical instruments and methods, molecular sieve catalysts, physical/chemical process catalysts, etc., can solve the problems of difficulty in improving the selectivity of reaction products and high pressure, and achieve hydrogenation rate enhancement, Avoid the effects of over-hydrogenation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
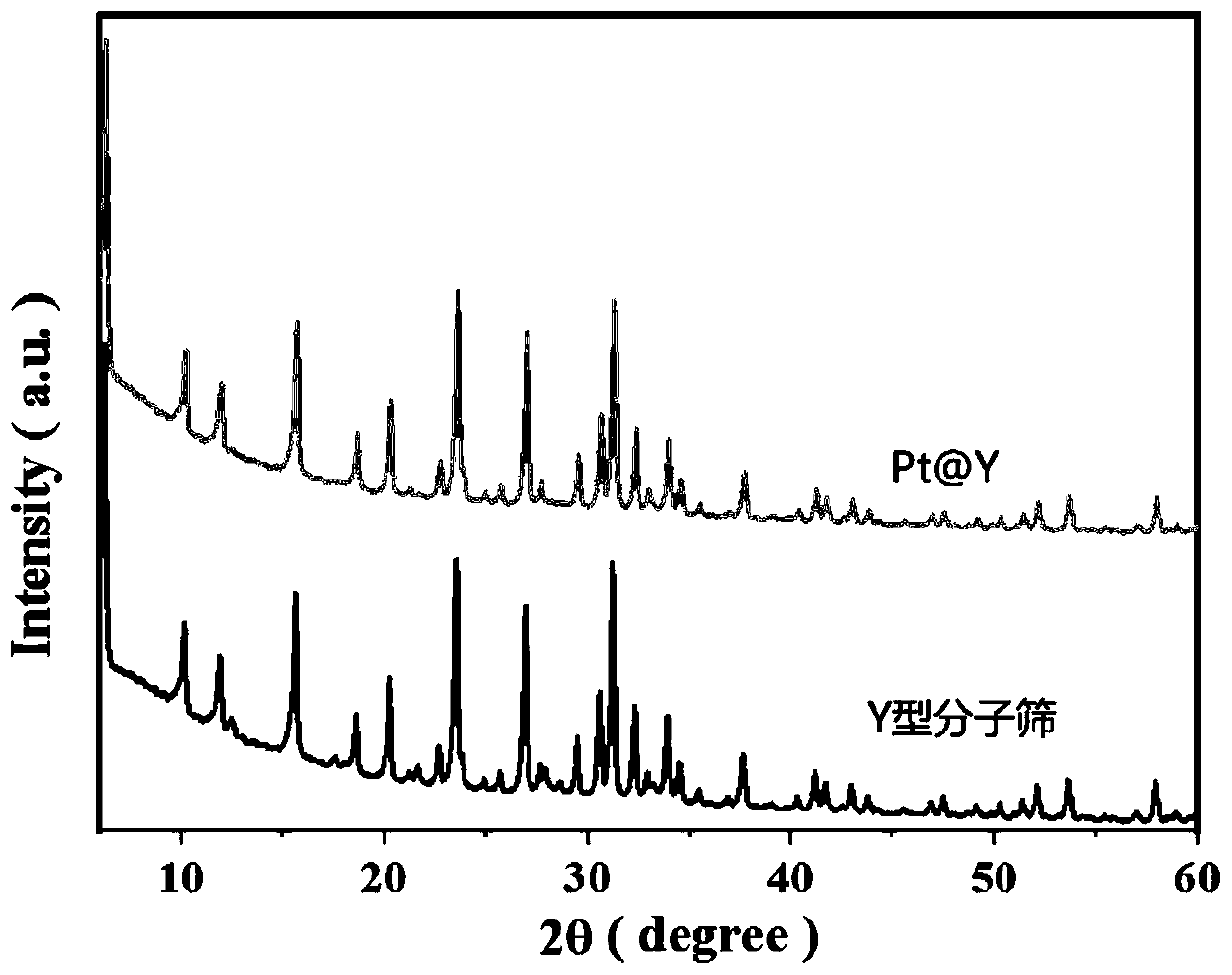
[0031] In this example, under a hydrogen atmosphere, 5-hydroxymethylfurfural is catalytically hydrogenated using nano-platinum encapsulated by Y molecular sieves as a catalyst to prepare 2,5-furandimethanol, which includes the following steps:
[0032] (1) Preparation of catalyst
[0033] Dissolve 4.44g of sodium hydroxide, 1.04g of aluminum hydroxide and 12.0g of Ludox-HS 30 in 40.0g of water, then add 0.1g of tetraaminoplatinum nitrate and stir evenly. The resulting mixture was aged at room temperature for 24 hours, then statically crystallized at 100° C. for 12 hours, then suction filtered and dried to obtain a white material.
[0034] The white material was placed in a muffle furnace, raised from room temperature to 350°C at a heating rate of 0.0114°C / s, and calcined at 350°C in an air atmosphere for 3 hours. 2 / N 2 Under mixed gas atmosphere (because all hydrogen treatment operations are particularly dangerous, use N 2 Dilution, H 2 The volume concentration in the mix...
Embodiment 2
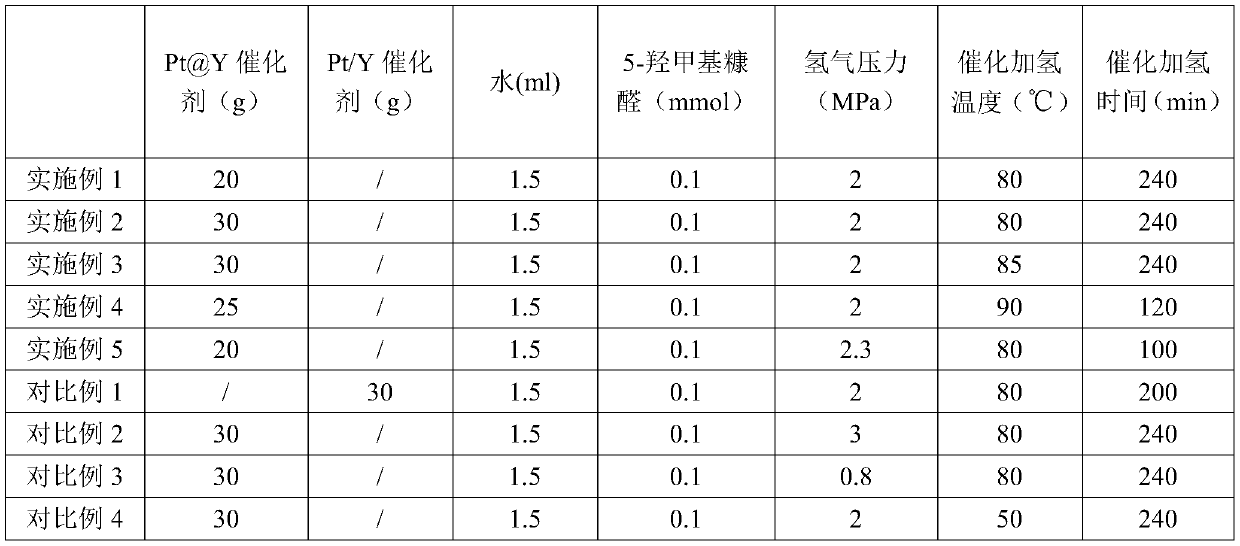
[0039] Put 30 mg of the Pt@Y catalyst prepared in Example 1 into a small autoclave, add 1.5 ml of water, and take 0.1 mmol of the substrate 5-hydroxymethylfurfural into the autoclave. The air in the autoclave was replaced with hydrogen for three times, then the pressure of hydrogen was increased to 2 MPa, and the reaction was carried out at 80° C. for 240 min. After the reaction was completed, the product was obtained by centrifugation and rotary evaporation. The isolated Pt@Y catalyst was washed three times with ethanol for the next use.
Embodiment 3
[0041] Put 30mg of the Pt@Y catalyst prepared in Example 1 into a small autoclave, add 1.5ml of water, take the substrate 5-hydroxymethylfurfural 0.1mmol into the autoclave, replace the air in the autoclave with hydrogen three times, The pressure was raised to 2MPa, and the reaction was carried out at 85°C for 240min. After the reaction was completed, the product was obtained by centrifugation and rotary evaporation. The isolated Pt@Y catalyst was washed three times with ethanol for the next use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com