Organic compound, thermally-activated delayed fluorescence material and application of organic compound
An organic compound and selected technology, applied in the field of organic compounds and thermally activated delayed fluorescent materials, can solve the problems that the performance is difficult to meet high-performance OLED devices, less TADF materials, etc., to improve carrier balance, improve transmission capacity, and improve The effect of device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
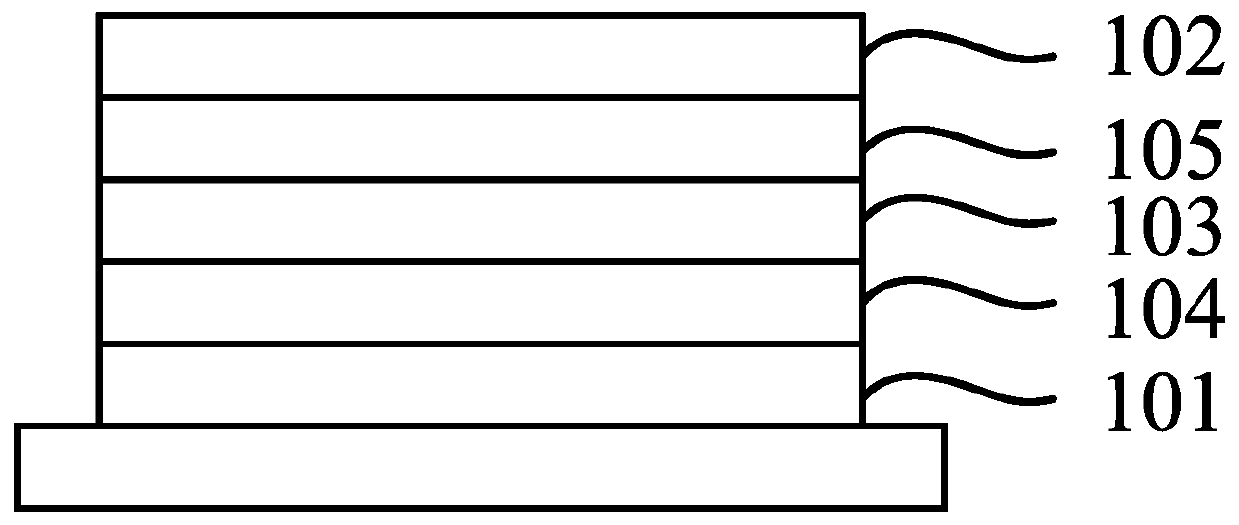
Image
Examples
Embodiment 1
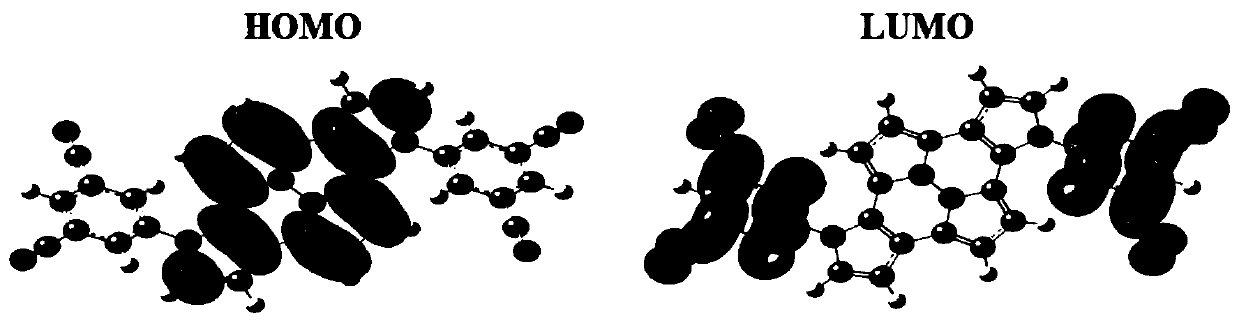
[0107] This embodiment provides an organic compound with the following structure:
[0108]
[0109] The preparation method of this organic compound M1 is:
[0110]
[0111] Add compound 1A (5.36g, 20mmol), compound 1B (11.24g, 40mmol), 150mL of toluene, cesium carbonate (13.03g, 40mmol) and tetrakis(triphenylphosphine) palladium to a 250mL three-necked flask. Pd(PPh 3 ) 4 (0.23g, 0.2mmol) and tri-tert-butylphosphine P(t-Bu) 3 (40.5mg, 0.2mmol), and then reacted at 120°C for 24h under nitrogen atmosphere. After being cooled to room temperature, the reaction solution was poured into 200 mL of ice water, extracted three times with dichloromethane, the organic phases were combined, and the solvent was removed by rotary evaporation, and silica gel column chromatography (the eluent was a mixture of dichloromethane / n-hexane with a volume ratio of 1:1) solution) separation and purification to obtain the target product M1.
[0112] The characterization results of the organic...
Embodiment 2
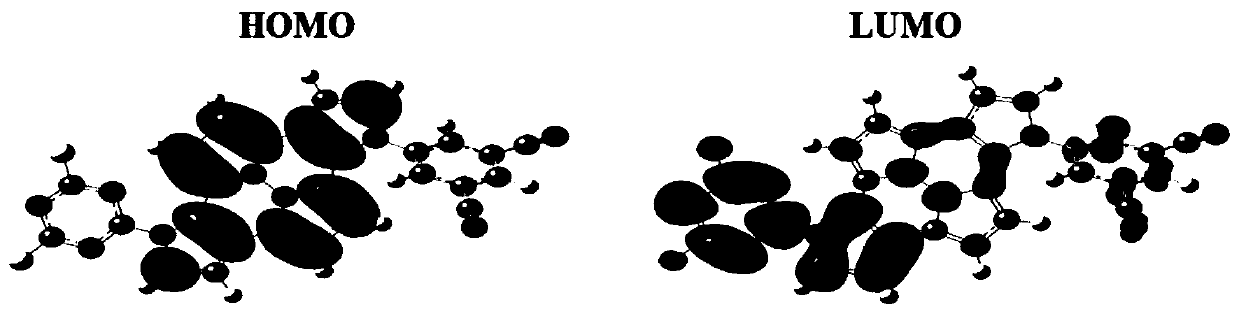
[0116] This embodiment provides an organic compound with the following structure:
[0117]
[0118] The preparation method of this organic compound M2 is:
[0119]
[0120] Add compound 1A (5.36g, 20mmol), compound 2B (5.62g, 20mmol), compound 2C (5.41g, 20mmol), 150mL toluene and cesium carbonate (13.03g, 40mmol) in a 250mL three-necked flask , Pd(PPh 3 ) 4 (0.23g, 0.2mmol) and P(t-Bu) 3 (40.5mg, 0.2mmol), and then reacted at 120°C for 24h under nitrogen atmosphere. Cooled to room temperature, the reaction solution was poured into 200mL of ice water, extracted three times with dichloromethane, the organic phases were combined, and the solvent was removed by rotary evaporation, and silica gel column chromatography (the eluent was a mixture of dichloromethane / n-hexane with a volume ratio of 1:1) solution) separation and purification to obtain the target product M2.
[0121] The characterization results of the organic compound M2:
[0122] 1 H-NMR (400MHz, CDCl 3 ): ...
Embodiment 3
[0125] This embodiment provides an organic compound with the following structure:
[0126]
[0127] The preparation method of this organic compound M3 is:
[0128]
[0129] Add compound 1A (5.36g, 20mmol), compound 3B (7.84g, 20mmol), compound 3C (5.24g, 20mmol), 150mL toluene and cesium carbonate (13.03g, 40mmol) in a 250mL three-necked flask , Pd(PPh 3 ) 4 (0.23g, 0.2mmol) and P(t-Bu) 3 (40.5mg, 0.2mmol), and then reacted at 120°C for 24h under nitrogen atmosphere. Cooled to room temperature, the reaction solution was poured into 200mL of ice water, extracted three times with dichloromethane, the organic phases were combined, and the solvent was removed by rotary evaporation, and silica gel column chromatography (the eluent was a mixture of dichloromethane / n-hexane with a volume ratio of 1:1) solution) separation and purification to obtain the target product M3.
[0130] The characterization result of the organic compound M3:
[0131] 1 H-NMR (400MHz, CDCl 3 ):...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap