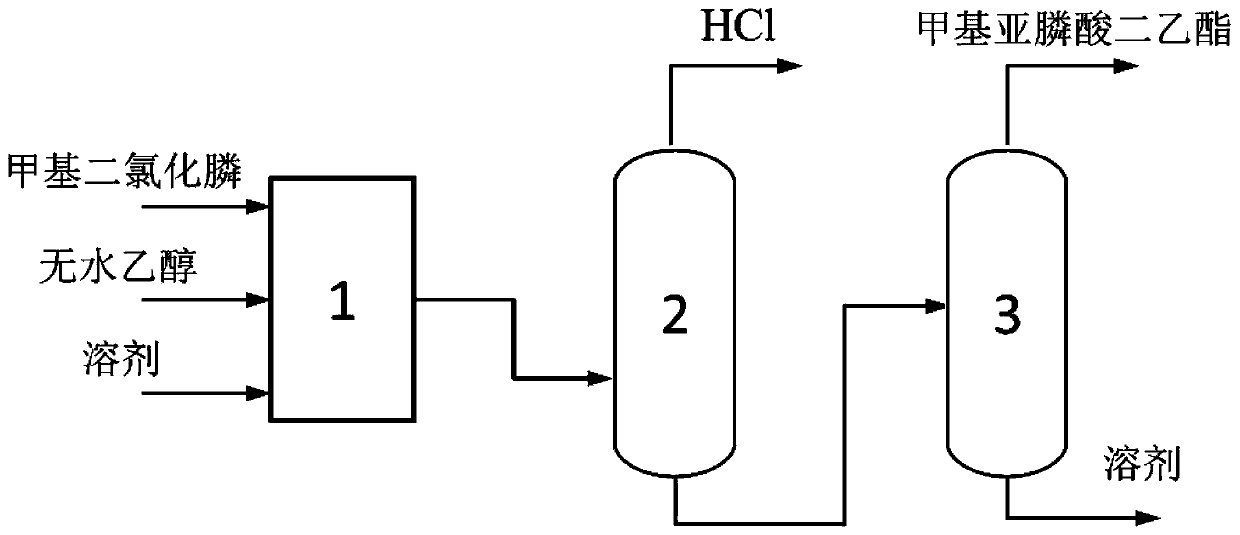
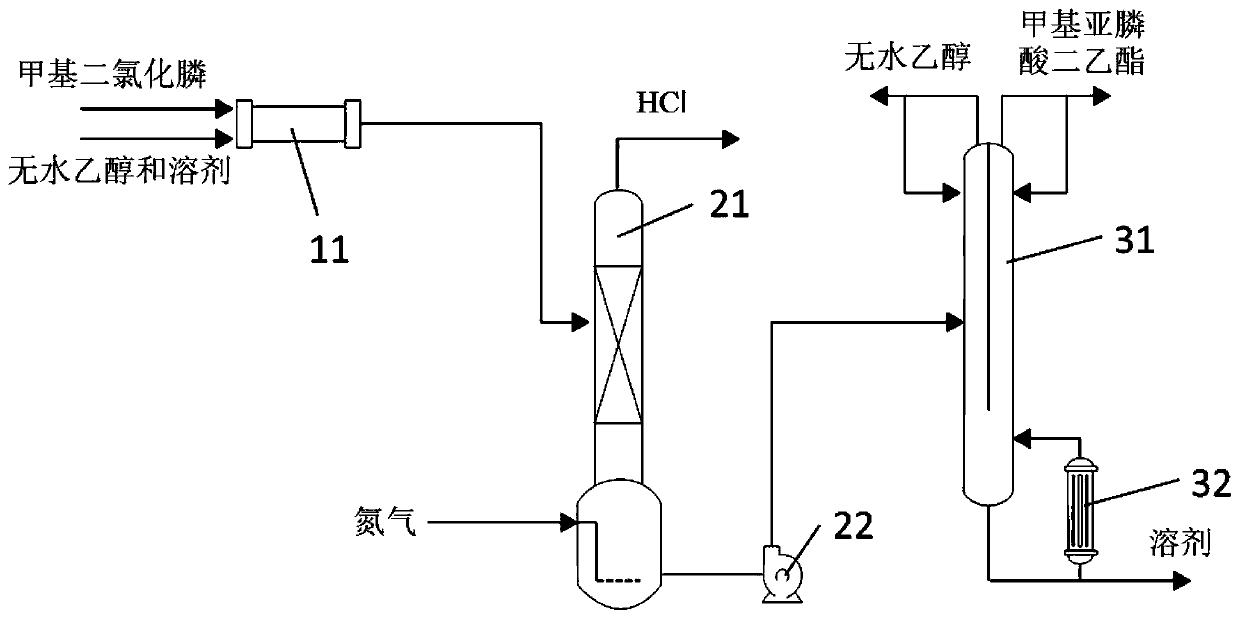
Synthesis method and synthesis device of diethyl methylphosphite
A technology of diethyl methyl phosphinate and a synthesis method is applied in the field of a synthesis method of diethyl methyl phosphinate and a synthesis device thereof, and can solve side reactions, high requirements on reaction process control, and synthesis reaction temperature. Advanced problems, to achieve the effect of reducing dosage, reducing solvent entrainment, reducing separation and processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The present embodiment provides a kind of synthetic method of diethyl methylphosphonite, and described synthetic method comprises the following steps:
[0073] (1) According to the molar ratio of methyl phosphine dichloride, dehydrated alcohol and solvent is 1:1.8:2, first the dehydrated alcohol of purity 99% and the decane of purity 99% are mixed, then mixed solution and purity 98% of methyl phosphine dichloride is mixed for continuous synthesis reaction, wherein the temperature of the synthesis reaction is controlled at -20~-10°C, and the time is 1s;
[0074] (2) The reaction solution obtained in step (1) is subjected to deacidification under reduced pressure, and nitrogen gas is passed to the bottom of the reaction solution at the same time, wherein, the pressure of the deacidification under reduced pressure is controlled to be -0.01MPa, and the temperature is -20 to -10°C , the average residence time is 60min;
[0075] (3) The deacidification reaction solution obta...
Embodiment 2
[0081] The present embodiment provides a kind of synthetic method of diethyl methylphosphonite, and described synthetic method comprises the following steps:
[0082] (1) According to the mol ratio of methyl phosphine dichloride, dehydrated alcohol and solvent is 1:1.8:5, first the dehydrated alcohol of purity 99% and the undecane of purity 99% are mixed, then mixed solution and Methylphosphine dichloride with a purity of 98% is mixed for a continuous synthesis reaction, wherein the temperature of the synthesis reaction is controlled at 10-20°C and the time is 0.01s;
[0083] (2) The reaction solution obtained in step (1) is subjected to deacidification under reduced pressure, and nitrogen is passed to the bottom of the reaction solution at the same time, wherein, the pressure of the deacidification under reduced pressure is controlled to be -0.03MPa, and the temperature is 10 to 20° C. The residence time is 30min;
[0084] (3) The deacidification reaction solution obtained i...
Embodiment 3
[0086] The present embodiment provides a kind of synthetic method of diethyl methylphosphonite, and described synthetic method comprises the following steps:
[0087] (1) According to the molar ratio of methylphosphine dichloride, absolute ethanol and solvent being 1:2:4, first mix absolute ethanol with a purity of 99% and 1,2,4-trimethylbenzene with a purity of 98%, Then, the mixed solution is mixed with methylphosphine dichloride with a purity of 98% to carry out a continuous synthesis reaction, wherein the temperature of the synthesis reaction is controlled to be 0-10°C, and the time is 0.5s;
[0088] (2) The reaction solution obtained in step (1) is subjected to deacidification under reduced pressure, and nitrogen gas is passed to the bottom of the reaction solution at the same time, wherein, the pressure of the deacidification under reduced pressure is controlled to be -0.06MPa, and the temperature is 0 to 10°C. Residence time 100min;
[0089] (3) The deacidification rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com