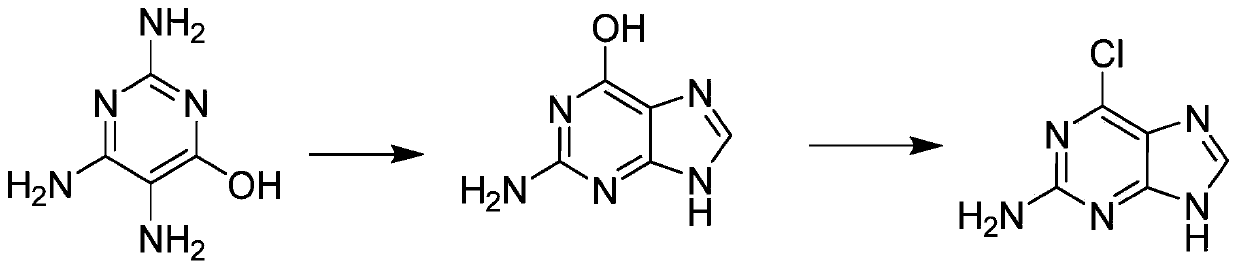
Method for synthesizing 2-amino-6-chloropurine
A technology of chloropurine and amino, which is applied in the field of synthesizing 2-amino-6-chloropurine, can solve the problems of low atom economy, many three waste products, strong corrosion, etc., and achieve short reaction time, high yield, and reaction The effect of fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A method for synthesizing 2-amino-6-chloropurine, comprising the steps of:
[0023] The first step: add 6-hydroxyl-2,4,5-triaminopyrimidine (120g) and trimethyl orthoformate (99.3g) into the reaction flask, slowly add 32% concentrated hydrochloric acid (145g) dropwise, and stir at room temperature 8 hours; add water (150g), neutralize pH7-8 with the liquid caustic soda of 2.5M, gained solid is filtered; At 40-45 degree, filter cake is beaten with water 1 hour; Filter and dry again, get white solid guanine ( 109g); second step: add guanine (100g) to 150.8g of 32% hydrochloric acid, control the temperature at 40~50°C, slowly add 30% hydrogen peroxide 75.1g dropwise, control the temperature at 40~50°C for 3 hours, and cool to 20 ℃ with 2.5M liquid caustic soda to neutralize the pH 6-7.5, filter with suction, and dry the product 2-amino-6-chloropurine (95.6g).
Embodiment 2
[0025] A method for synthesizing 2-amino-6-chloropurine, comprising the steps of:
[0026] The first step: 6-hydroxyl-2,4,5-triaminopyrimidine (120g) and trimethyl orthoformate (99.3g) were added in the reaction flask, slowly added dropwise 32% acetic acid (145g), stirred at room temperature for 8 Hour; Add water (150g), neutralize pH7-8 with the liquid caustic soda of 2.5M, gained solid is filtered; At 40-45 degree, filter cake is beaten with water 1 hour; Filter and dry again, get white solid guanine (109g ); second step: add guanine (100g) to 150.8g of 32% hydrochloric acid, control the temperature at 40-50°C, slowly add 30% hydrogen peroxide 75.1g dropwise, control the temperature at 40-50°C for 3 hours, and cool to 20°C Use 2.5M liquid caustic soda to neutralize pH 6-7.5, filter with suction, and dry the product 2-amino-6-chloropurine (94.1g).
Embodiment 3
[0028] A method for synthesizing 2-amino-6-chloropurine, comprising the steps of:
[0029] The first step: add 6-hydroxyl-2,4,5-triaminopyrimidine (120g) and trimethyl orthoformate (99.3g) into the reaction flask, slowly add 32% acetic anhydride (145g) dropwise, and stir at room temperature 8 hours; add water (150g), neutralize pH7-8 with the liquid caustic soda of 2.5M, gained solid is filtered; At 40-45 degree, filter cake is beaten with water 1 hour; Filter and dry again, get white solid guanine ( 109g); second step: add guanine (100g) to 150.8g of 32% hydrochloric acid, control the temperature at 40~50°C, slowly add 30% hydrogen peroxide 75.1g dropwise, control the temperature at 40~50°C for 3 hours, and cool to 20 ℃ with 2.5M liquid caustic soda to neutralize pH 6-7.5, filter with suction, and dry the product 2-amino-6-chloropurine (93.6g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com