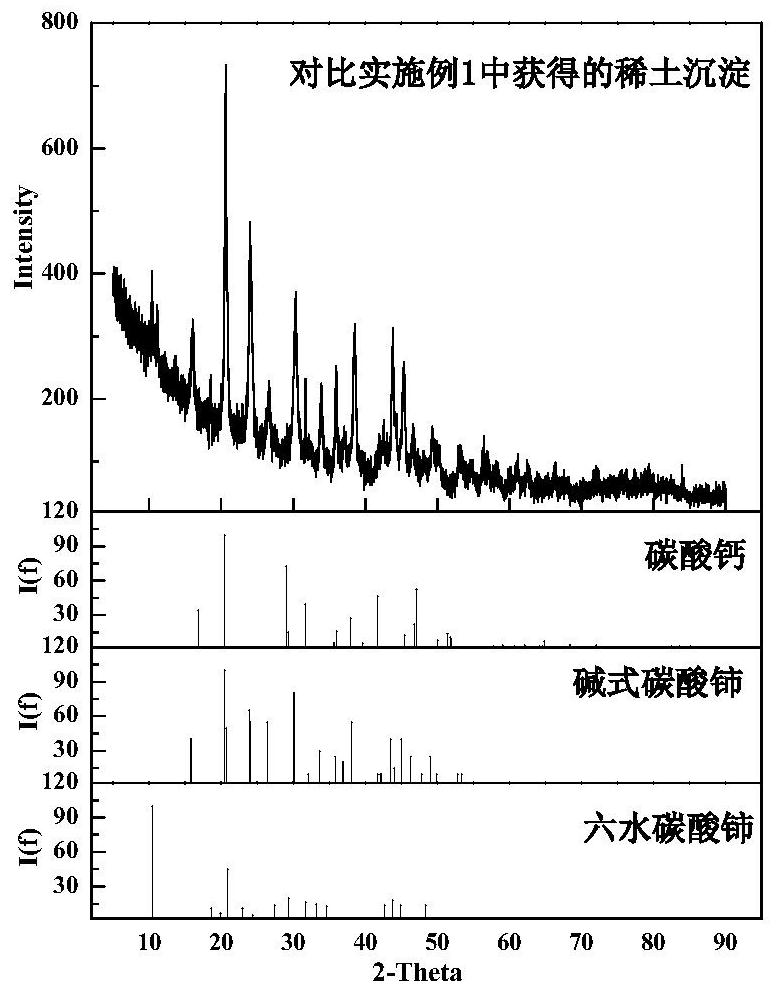
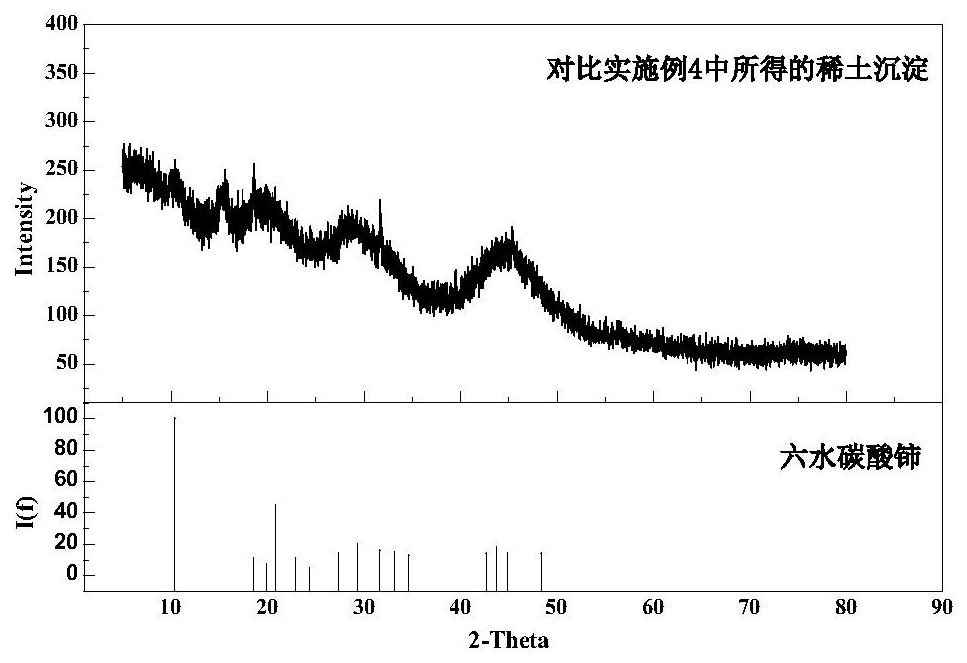
A method for precipitation and separation of rare earth in calcium-containing rare earth solution
A rare earth solution, precipitation separation technology, applied in the field of precipitation and separation of rare earth and calcium, can solve the problems of instability, inability to achieve calcium rare earth separation, difficult to convert calcium carbonate into calcium bicarbonate, etc., and achieve the effect of efficient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
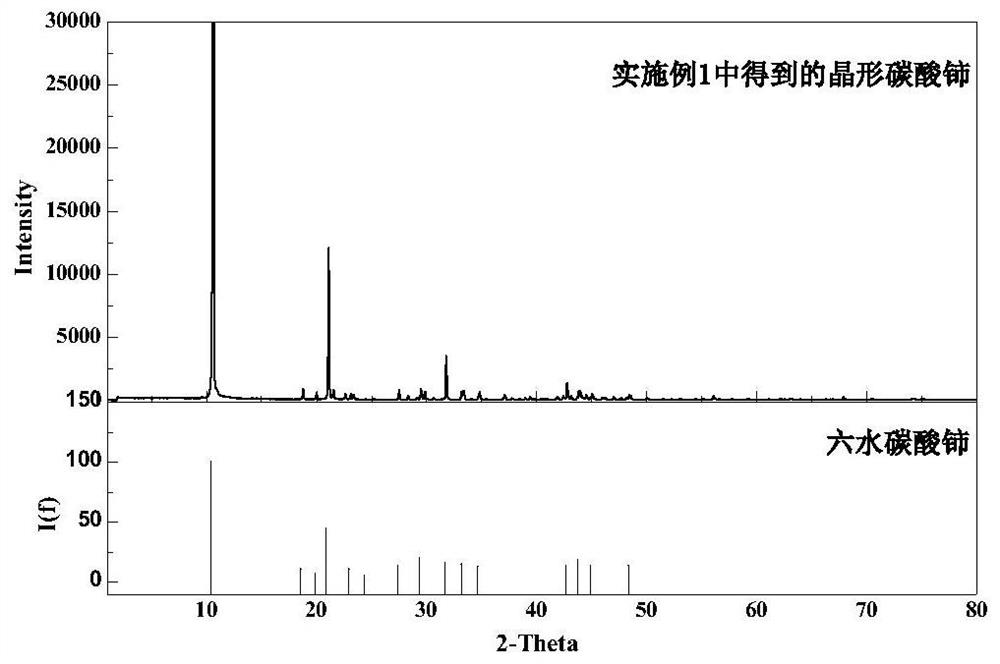
[0042] First, add bottom water and cerium carbonate seed crystals to the reaction kettle, and then add a calcium-containing rare earth solution with a cerium ion concentration of 0.3 mol / L and a calcium ion concentration of 0.30 mol / L and a sodium bicarbonate solution of 0.30 mol / L in parallel Carry out the precipitation reaction, control the reaction temperature to 25°C, control the feeding rate of the calcium-containing rare earth solution to 2.0ml / min, adjust the feeding rate of the sodium bicarbonate solution to control the pH of the reaction process to be 4.6, filter and wash after the reaction to obtain crystalline carbonic acid Cerium precipitate and filtrate; crystalline cerium carbonate phase as image 3 As shown in XRD, it can be seen that it is cerium carbonate hexahydrate with high purity and good crystallinity. The cerium carbonate precipitate was calcined at 900°C for 3 hours to obtain ceria with a purity of 99.0%.
Embodiment 2
[0044] First add the bottom water in the reactor, and then add the calcium-containing rare earth solution and the sodium bicarbonate solution of 0.30 mol / L with the cerium ion concentration of 0.3mol / L and the calcium ion concentration of 0.30mol / L in parallel to carry out the precipitation reaction. The reaction temperature is 25°C, the feeding rate of the calcium-containing rare earth solution is controlled to be 2.0ml / min, the pH of the reaction process is controlled to be 4.6 by adjusting the feeding rate of the sodium bicarbonate solution, and the crystalline cerium carbonate precipitate and filtrate are obtained by filtering and washing after the reaction , The cerium carbonate precipitate was calcined at 900°C for 3h to obtain ceria with a purity of 98.2%.
Embodiment 3
[0046] First, add bottom water and cerium carbonate seed crystals to the reaction kettle, and then add a calcium-containing rare earth solution with a cerium ion concentration of 1.2 mol / L and a calcium ion concentration of 0.20 mol / L and a sodium bicarbonate solution of 0.80 mol / L in parallel Carry out the precipitation reaction, control the reaction temperature to 40°C, control the feeding rate of the calcium-containing rare earth solution to 7.0ml / min, adjust the feeding rate of the sodium bicarbonate solution to control the pH of the reaction process to be 5.1, filter and wash after the reaction to obtain the crystal form Cerium carbonate precipitate and filtrate, the cerium carbonate precipitate was calcined at 900°C for 3 hours to obtain cerium oxide with a purity of 99.2%. The physical properties of cerium oxide are as follows: Figure 4 As shown, it is a pure ceria phase with no miscellaneous peaks and high purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com