Preparation method and application of anti-tumor vaccine antigen raw material
A vaccine antigen, anti-tumor technology, applied in anti-tumor drugs, biochemical equipment and methods, cancer antigen components, etc., can solve the problems of immune escape, complex extraction and preparation of tumor cell antigen composition, etc. The effect of simple immune escape, extraction and preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation and preservation method of anti-tumor vaccine antigen raw material
[0032] Cultivate tumor cells with 10% FBS (fetal bovine serum) medium in a 10mm×10mm petri dish until the cells in the dish reach about 5×10 6 At one hour, X-rays were used to irradiate with a dose of 20GY. The cells in the culture dish were collected on the third day after the irradiation. After the cells were broken, they were centrifuged by gradient centrifugation. Centrifuge at 14000g for 60min, discard the supernatant, and the resulting precipitate is the tumor vaccine antigen raw material.
[0033] The obtained precipitate was washed twice with normal saline, resuspended in 1ml PBS (phosphate buffered saline) solution, and stored at 4°C. After centrifuging 100 μl of the liquid, an appropriate amount of protein lysate was added, fully lysed at 0°C for 30 minutes, and then centrifuged at 12,000 g for 30 minutes. The supernatant was taken and added to BCA quantitative solutio...
Embodiment 2
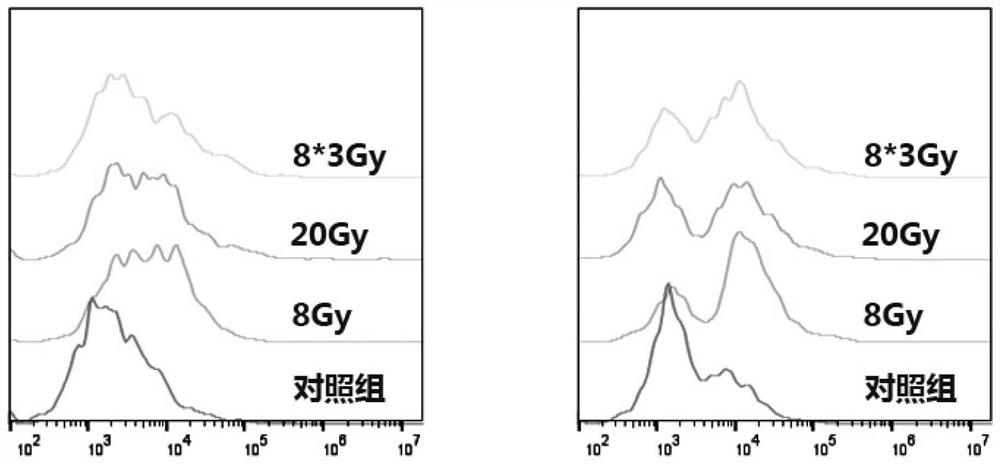
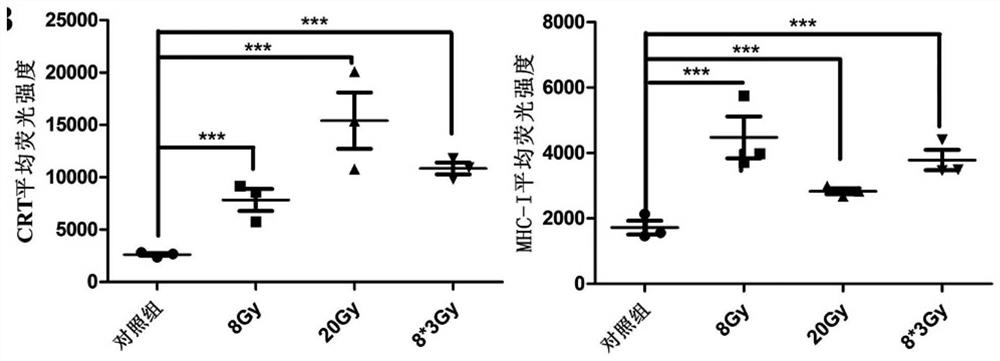
[0034] Example 2: Expression experiment of MHC I and CRT on the surface of anti-tumor vaccine antigen raw materials.
[0035] Experimental steps:
[0036] (1) Mouse-derived lung adenocarcinoma cells Lewis received X-rays 8Gy, 20Gy, 8*3Gy;
[0037] (2) Add trypsin to digest each group of cells into a single cell suspension and collect them in EP tubes;
[0038] (3) Centrifuge in a low-speed centrifuge at a speed of 1000rpm / 5min, discard the supernatant after centrifugation, add 1mL of PBS buffer, mix well and centrifuge again at a speed of 1000rpm / 5min for 3 times, discard the supernatant, and use 200μL of PBS buffer Resuspend, add to the flow tube and mark it;
[0039](4) Add 1 μ antibody (CRT:FITC, MHC-I:FITC) to each flow tube and incubate at 4°C in the dark for 20 minutes;
[0040] (5) Add 1mL of PBS buffer, mix well, centrifuge 3 times at 1000rpm / 5min, discard the supernatant, add 200μ PBS to resuspend, and test on the machine.
[0041] Experimental conclusion: 20Gy ra...
Embodiment 3
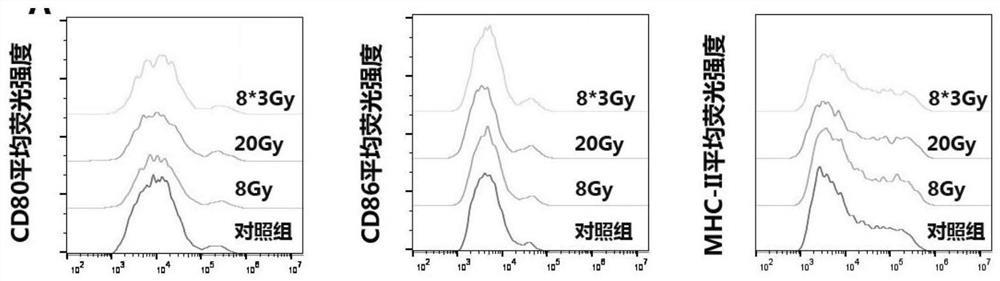
[0042] Example 3: Experiment on the stimulating effect of anti-tumor vaccine antigen raw materials on bone marrow-derived dendritic cells.
[0043] Experimental steps:
[0044] (1) Mouse-derived lung adenocarcinoma cells, Lewis, were plated on a 6-well plate and irradiated with 8Gy, 20Gy, and 8*3Gy;
[0045] (2) Extract the cells of the control group and each radiotherapy group, use ultrasonic means to break the cells, and then centrifuge at a speed of 2000rpm / 10min, discard the precipitate and take the supernatant, and centrifuge at 10000rpm for 60min to obtain the precipitate, which is the tumor cell membrane;
[0046] (3) extract mouse bone marrow-derived mononuclear cells and differentiate them with GM-CSF to induce the formation of DC cells;
[0047] (4) Add the tumor cell membrane extracted from every 1 million tumor cells to 500,000 DC cells for co-culture for 6 hours, and then collect the DC cells into a clean EP tube;
[0048] (5) Centrifuge in a low-speed centrifug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com