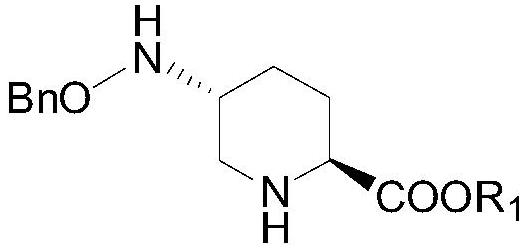
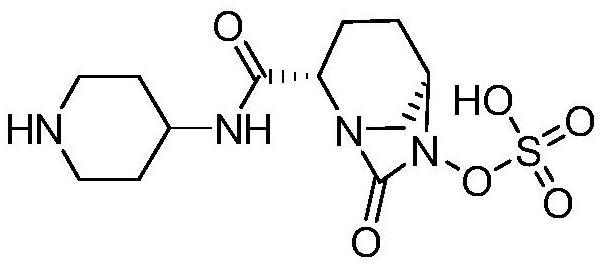
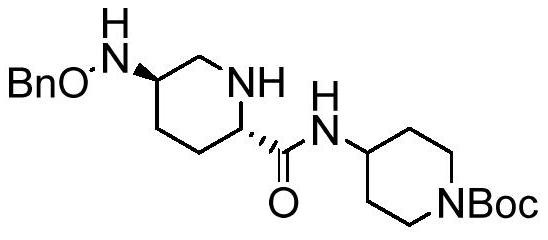
Preparation method of Rayleigh bactam intermediate
A technology for intermediates and compounds, applied in the field of pharmaceutical synthesis, can solve the problems of special raw materials, low reaction conversion rate, increase cost, etc., and achieve the effects of mild reaction conditions, improved reaction yield, and reduced preparation cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: (R1 is benzyl)
[0033] Put 30ml of toluene, 0.7g (5.3mmol) of anhydrous aluminum chloride, 7.0g (35mmol) of compound III, 5g (14.7mmol) of compound II into the reaction bottle, keep warm at 50°C until the reaction is complete (about 3 hours), add 50% Sodium hydroxide aqueous solution 2.0g, filter, separate layers, depressurize the organic phase to dryness, crystallize with petroleum ether, filter with suction, rinse and dry the filter cake with petroleum ether to obtain 5.6g of compound I, yield: 88%, Content: 98.5%. 1 H NMR (400MHz, CDCl 3 )δ7.40–7.27(m,5H),6.75(d,J=8.0Hz,1H),4.66(s,2H),4.00(s,2H),3.93–3.82(m,1H),3.29(dd ,J=11.8,2.4Hz,1H),3.16(dd,J=10.4,2.8Hz,1H),2.96(t,J=10.0Hz,1H),2.84(t,J=11.5Hz,2H),2.53 –2.42(m,1H),2.15–2.05(m,1H),1.88(dd,J=23.3,8.8Hz,3H),1.44(d,J=3.5Hz,10H),1.38–1.20(m,3H ). Ms: M+1=433.28.
[0034] Reduce the mother liquor to dryness, add 30ml of toluene, 3.8g (19mmol) of compound III, 0.7g (5.3mmol) of anhydrous aluminum chl...
Embodiment 2
[0035] Embodiment 2: (R1 is benzyl)
[0036] Put 30ml of acetonitrile, 0.7g (5.3mmol) of anhydrous aluminum chloride, 7.0g (35mmol) of compound III, 5g (14.7mmol) of compound II into the reaction bottle, keep warm at 50°C until the reaction is complete, add 2.0% 50% sodium hydroxide aqueous solution g, filter, decompress the filtrate to dryness, crystallize with cyclohexane, filter with suction, rinse the filter cake with pure water, and dry to obtain 5.5g of compound I, yield: 86%, content: 99.0%.
[0037] Reduce the mother liquor to dryness, add 30ml of acetonitrile, 3.8g (19mmol) of compound III, 0.7g (5.3mmol) of anhydrous aluminum chloride, 5g (14.7mmol) of compound II, keep warm at 50°C until the reaction is complete, add 50% hydroxide Sodium aqueous solution 2.0g, filtered, separated, the organic phase was decompressed to dryness, crystallized with cyclohexane, filtered with suction, and the filter cake was rinsed and dried with cyclohexane to obtain 6.0g of compound I,...
Embodiment 3
[0038] Embodiment 3: (R1 is benzyl)
[0039] Put 30ml of toluene, 1.02g (7.5mmol) of anhydrous zinc chloride, 7.0g (35mmol) of compound III, 5g (14.7mmol) of compound II into the reaction bottle, keep warm at 50°C until the reaction is complete, add 2.0% 50% sodium hydroxide aqueous solution g, filter, separate layers, decompress the organic phase to dryness, crystallize with petroleum ether, filter with suction, and dry to obtain 5.08g of compound I, yield: 80%, content: 97.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com