Toxoplasma gondii attenuated live vaccine with deletion of AP2IV-1 gene and construction method thereof
A technology of AP2IV-1, 1. AP2IV-1 is applied in the construction method and the vaccine containing the attenuated parasite strain, the field of the attenuated parasite of Toxoplasma gondii, and can solve the problems of strong virulence, restricting the use of vaccines, etc. The effect of disease reduction, high immune protection efficacy, and reduced proliferation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
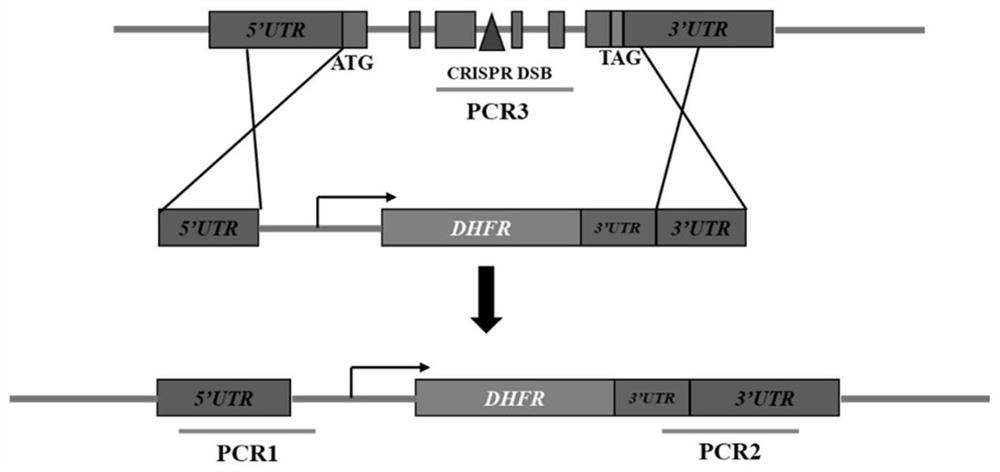
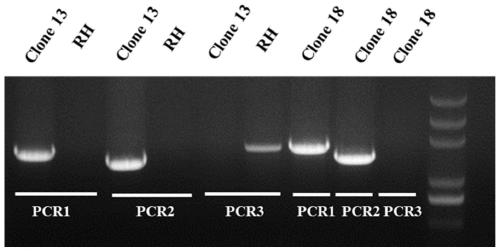
[0039] Example 1 Construction of Toxoplasma gondii AP2IV-1 gene deletion strain
[0040] The AP2IV-1 gene-deficient strain was constructed with ΔKu80 RH as the basic strain.
[0041] ΔKu80 RH is a Toxoplasma type I strain that loses the ability to form cysts in animals. The sequence of AP2IV-1 is conserved in different types of Toxoplasma gondii, its amino acid sequence is shown in SEQ ID NO.1, and its nucleotide sequence is shown in SEQ ID NO.2.
[0042] 1. Construction of CRISPR / CAS9 system plasmid pSAG1-CAS9-TgU6-sgAP2IV-1
[0043] Using the pSAG1-CAS9-TgU6-sgUPRT plasmid as a template, the multi-fragment seamless cloning kit from TransGen Biotech ( Seamless Cloning and Assembly Kit) to replace the gRNA of the UPRT gene with the gRNA of the AP2IV-1 gene. The specific steps are as follows:
[0044] (1) Design of gRNA
[0045] The gRNA of AP2IV-1 was designed in the ToxoDB (http: / / grna.ctegd.uga.edu / ) database of Toxoplasma gondii. Since the AP2IV-1 gene sequence is less...
Embodiment 2
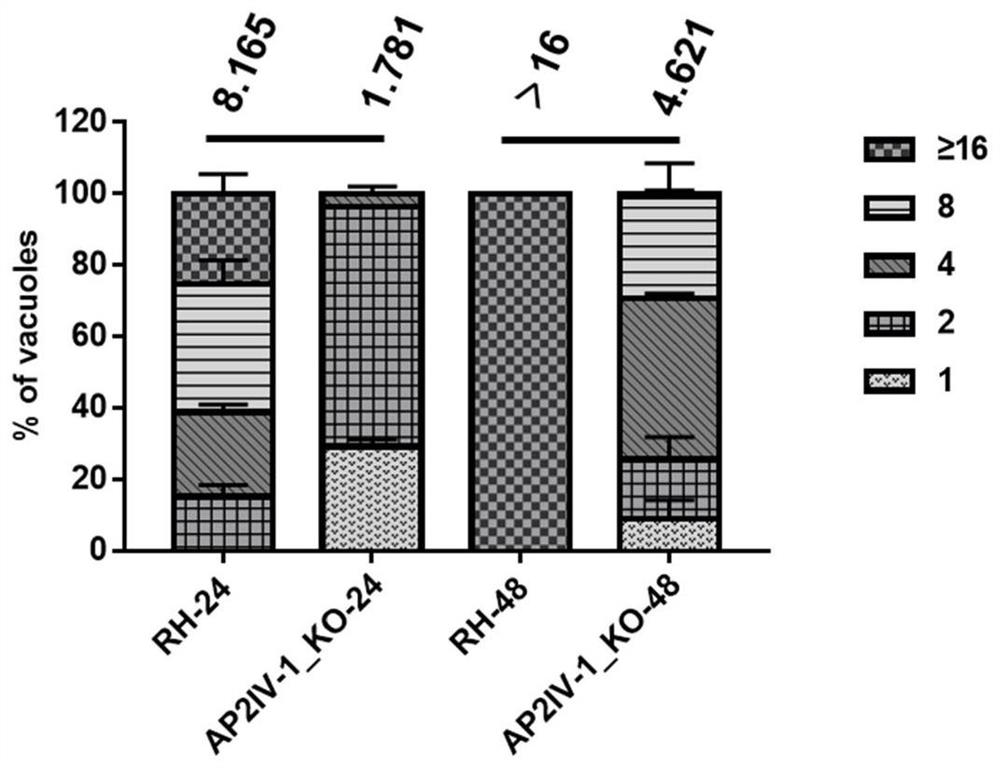
[0103] Example 2 Detection of proliferation rate, virulence and immune protection of Toxoplasma gondii AP2IV-1 gene-deleted strain
[0104] 1. In vitro proliferation experiment of RH ΔAP2IV-1 strain
[0105] The intracellular proliferation rate of the Toxoplasma gondii AP2IV-1 gene deletion strain RH ΔAP2IV-1 constructed in Example 1 was detected, and the specific method was as follows:
[0106] Tachyzoites of freshly released ΔKu80 RH and RH ΔAP2IV-1 strains were collected and inoculated for 10 5 The tachyzoites were placed in a 12-well plate filled with HFF cells (human foreskin fibroblasts, purchased from ATCC Company) (put a sterile cell slide before laying the cells). After 1 hour of inoculation, the uninvaded insects were washed away, and the cultivation was continued in the incubator. After culturing for 24h or 48h, carry out the IFA test, the specific method is as follows:
[0107] ① Fix the cells infected with Toxoplasma gondii in 4% paraformaldehyde at 37°C for 30...
Embodiment 3
[0129] Example 3 Monitoring of Humoral Immunity and Cellular Immune Response of RH ΔAP2IV-1 Strain Immunized Host
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com