Application of ASGR1 mutant gene in preparation of humanoid low-blood-fat metabolic animal model
A technique of mutating genes and animal models, applied in the field of genetic engineering, can solve the problems of high cost of animal preparation, difficulty in surviving, and high cost of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
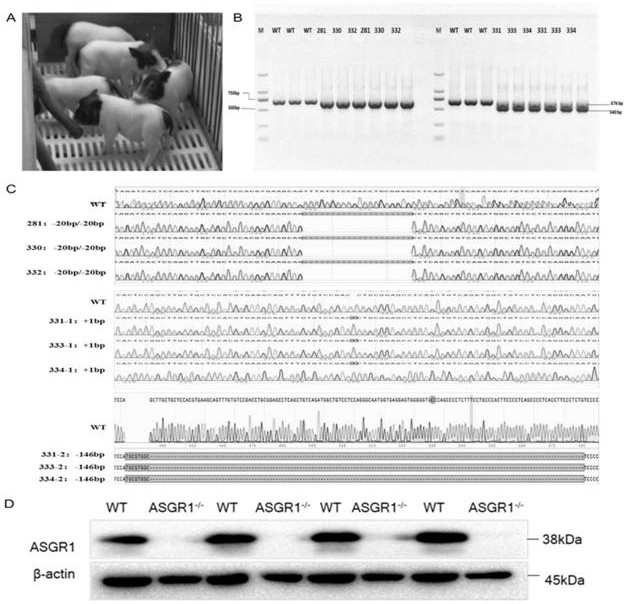
[0041] Embodiment 1, the preparation of ASGR1 gene knockout piglet
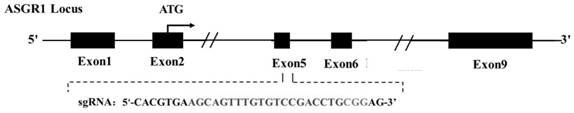
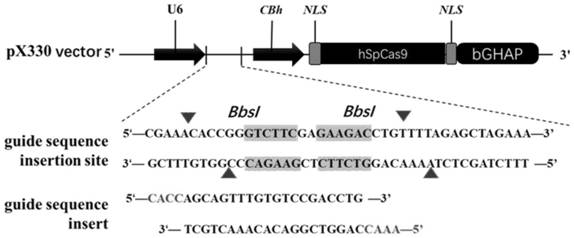
[0042] According to the pig (Sus scrofa) asialoglycoprotein receptor 1 (asialoglycoprotein receptor, ASGR1) gene sequence (Gene ID: NC_010454.4), using single-stranded guide RNA (single guide RNA, sgRNA) online design website (http: / / crispr.mit.edu / ) in its 5th exon ( figure 1 ) designed and synthesized sgRNA (sgRNA:agcagtttgtgtccgacctgcgg) that specifically recognizes the target sequence DNA. After the synthesized sgRNA oligonucleotides were annealed (94°C, 10min; 37°C, 10min), they were ligated into the PX330 expression vector recovered by digestion with BbsI to construct the sgRNA expression vector ( figure 2 ). After the constructed expression vector was sequenced to verify that the connection was correct, the plasmid was extracted for cell transfection.
[0043] The verified effective sgRNA expression vector and Enhanced Green Fluorescent Protein (Enhanced Green Fluorescent Protein, EGFP) plasmid we...
Embodiment 2
[0053] Example 2. Comparison of the similarity between the hypolipidemia phenotype of the ASGR1 gene knockout animal model and the ASGR1 mutant population
[0054] The ASGR1 knockout pigs obtained in Example 1 were tested for blood lipid indexes.
[0055] 1. Experimental animals
[0056] Experimental group 1: ASGR1-KO 3 heads (prepared in Example 1)
[0057] Experimental group 2: ASGR1-SKO pigs (individuals produced by breeding ASGR1-KO pigs with WT sows)
[0058] Control group: wild-type pigs (wide type, WT) without gene editing were used as controls.
[0059] All experimental animals were fed under the same experimental conditions.
[0060] 2. Test method:
[0061] At the same month age (17 months), fasting blood was collected from animals in the three groups, serum was separated, and blood lipid indicators (blood total cholesterol TC, triglyceride TG, LDL-c, HDL-c, Non-HDL-c, apoA1, apoB, etc.)
[0062] 3. Test results:
[0063] For ASGR1 knockout pigs (ASGR1-KO (ASG...
Embodiment 3
[0065] Example 3. Resistance of ASGR1 knockout animal model to high-fat and high-glucose-induced atherosclerosis
[0066] In the present invention, the tolerance experiment of atherosclerosis is carried out on the ASGR1 knockout pig and the control group.
[0067] A high-fat and high-cholesterol diet (20% fat, 2% cholesterol) was used to induce ASGR1 knockout pigs to model atherosclerosis with the control group. At the same time, a blank control group fed normal diet was set up to speed up the experimental process. The ASGR1 knockout pigs were fed for 6 months with a high-fat and high-cholesterol diet, and the high-fat and high-cholesterol diet and normal feed formulations are shown in Table 3.
[0068] Table 3 Test pig feed formula (%)
[0069] raw material name normal feed High Fat and High Cholesterol Feed corn 80.2 61.8 soybean meal 12 9.2 rice bran 6 4.7 fish meal 0.3 0.3 stone powder 0.72 0.7 salt 0.28 0.3 but...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com