Nano drug delivery system capable of delivering genetic drug towards secondary hepatocyte in targeted mode and application
A technology for gene delivery and drug delivery system, applied in the field of pharmacy, can solve problems such as low targeting efficiency, and achieve the effects of quality control, improved utilization, and long-term stable therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
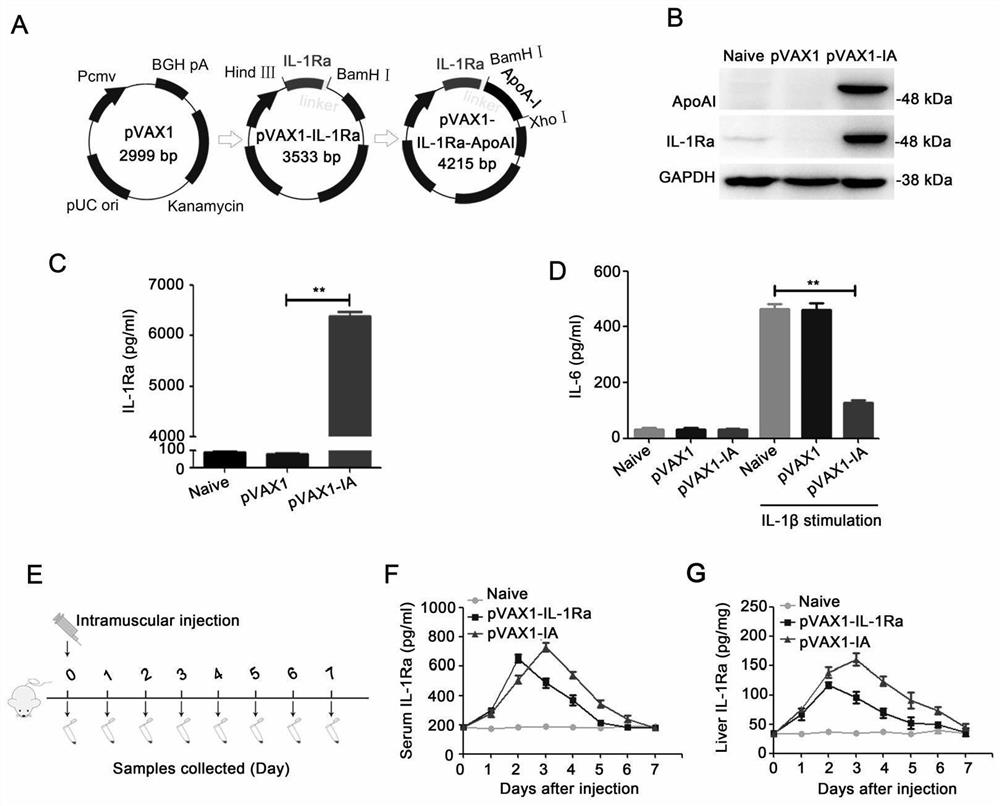
[0058] Example 1 Preparation of Interleukin 1 Receptor Antagonist (IL-1Ra) and Apolipoprotein AI (ApoAI) Fusion Gene surface delivery vehicle pVAX1-IL-1Ra-ApoAI (pVAX1-IA)
[0059] The gene sequences of IL-1Ra and ApoAI are derived from GeneBank accession no.NM_173842.2 and GeneBank accession no.X07496 respectively, and the protein sequence added between the gene sequences of IL-1Ra and ApoAI is a flexible short peptide of GGGGS (gene sequence: GGCGGAGGCGGATCC) Connection; the Kozak sequence was added to the upstream sequence of IL-1Ra as an enhancer; the gene sequences of IL-1Ra-GGGGS and ApoAI were synthesized by GenScript Biotechnology Company on the pMD19-Tsimple vector, and the two ends of IL-1Ra-GGGGS The restriction endonuclease cutting sites of HindIII and BamHI are designed, and the restriction endonuclease cutting sites of BamHI and XhoI are designed at both ends of ApoAI;
[0060] Take the pVAX1 expression vector and pMD19-T simple-IL-1Ra-GGGGS encoding gene pla...
Embodiment 2
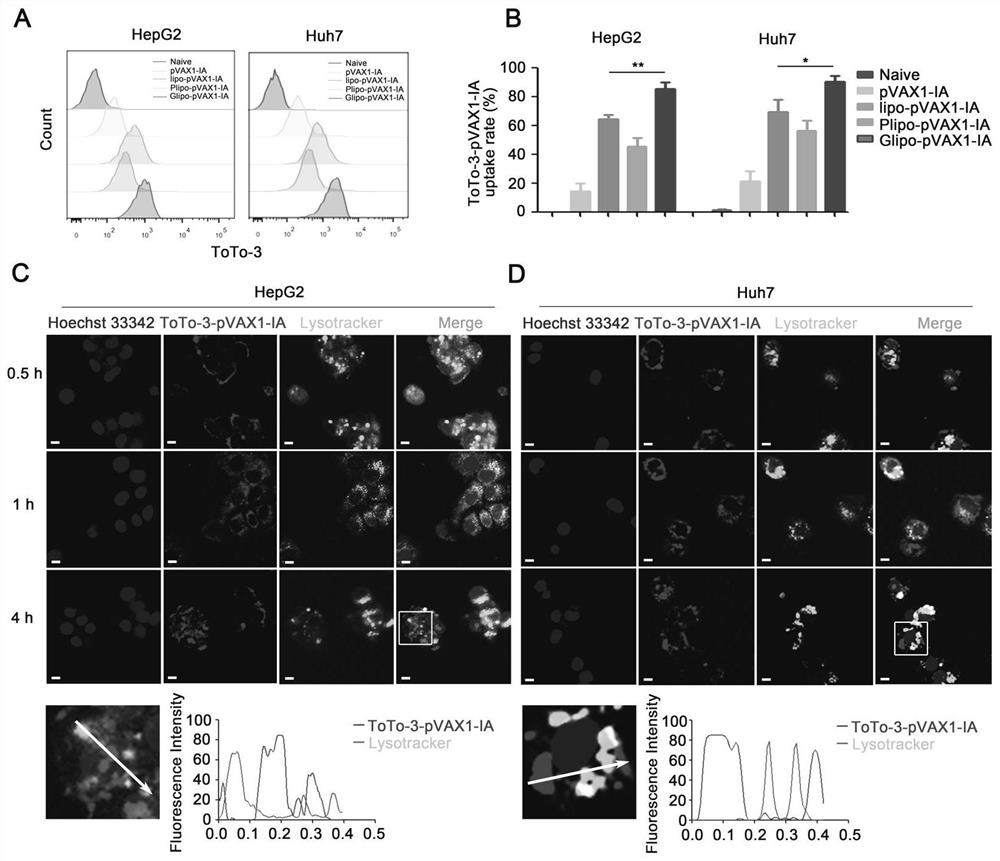
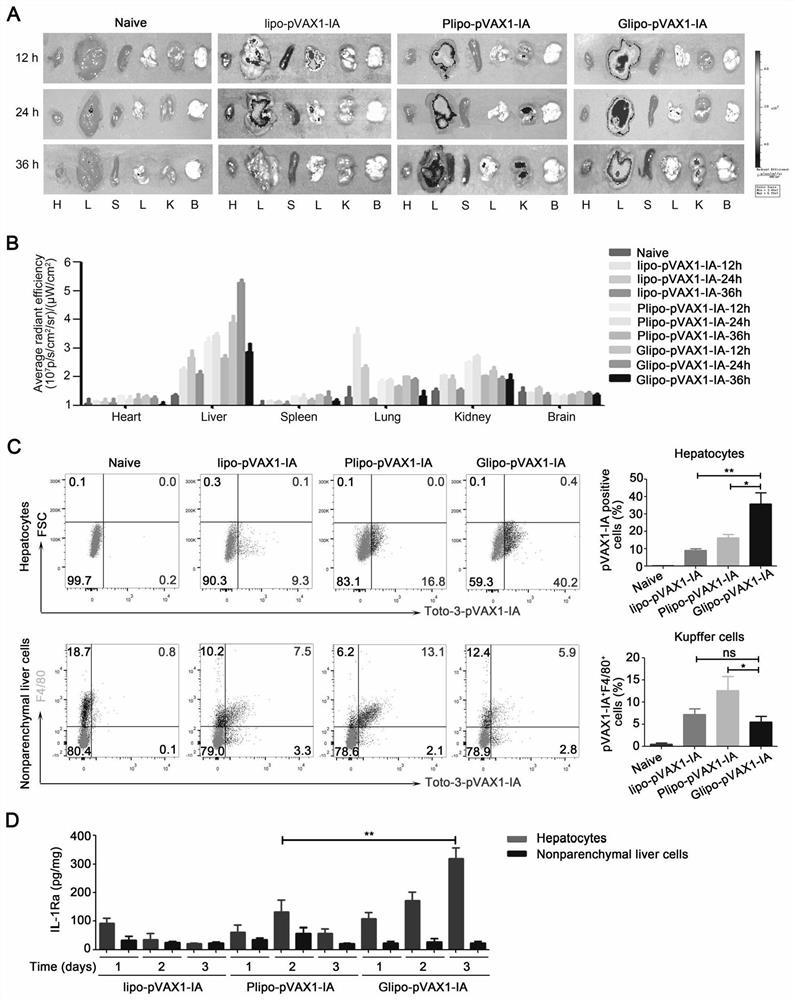
[0062] Example 2, the expression level of gene drug pVAX1-IA in vivo and in vitro
[0063] HEK-293T cells were planted in a six-well plate. When the cell fusion rate reached 70-80%, 5 μg of pVAX1-IA was transfected with lipofectamine2000, and the cells were cultured for 24 hours. Western blot and ELISA were used to detect intracellular and secreted IL-1Ra content in the supernatant, the results confirmed the successful expression and secretion of IL-1Ra-ApoAI fusion protein ( figure 1 B-C); In order to further verify the expression of pVAX1-IA in mice, C57BL / 6 mice were divided into three groups and injected intramuscularly with PBS, pVAX1-IL-1Ra and pVAX1-IA (the weight of the plasmid was 50 μg) respectively. The specified time detects the content of IL-1Ra in blood and liver of mice ( figure 1 E-G); As shown, pVAX1-IA prolongs the half-life of pVAX1-IL-1Ra in mice, and also increases the content of IL-1Ra in the liver.
Embodiment 3
[0064] Example 3. In vitro activity evaluation of gene drug pVAX1-IA
[0065] HEK-293T cells were planted in a six-well plate, and when the cell fusion rate reached 70-80%, 5 μg of pVAX1-IA was transfected with lipofectamine2000. After continuing to culture for 24 hours, the supernatant of HEK-293T cells transfected with pVAX1-IA was collected and applied to A549 cells. The increase of IL-6 in A549 cells caused by 100pg / ml IL-1β was significantly inhibited, proving that pVAX1-IA The expressed IL-1Ra-ApoAI fusion protein antagonized the activity of IL-1β ( figure 1 D).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com