Binuclear rare earth olefin polymerization catalyst and application thereof
A technology of olefin polymerization and catalyst, which is applied in the field of catalysts for binuclear rare earth olefin polymerization, and achieves the effect of high stereoregularity and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of mononuclear rare earth complex 1
[0040]
[0041] At 0°C, n-butyllithium was slowly added dropwise to the hexane solution of Cp*, then naturally warmed to room temperature and stirred overnight, resulting in a large amount of pale yellow precipitate. Subsequently, suction filtration was carried out in an inert gas glove box, the obtained solid was washed twice with hexane, and dried under vacuum to obtain a light yellow powder solid which was the Cp*Li compound. At room temperature, the THF solution of Cp*Li was slowly added dropwise to YCl 3 .nTHF in THF suspension, then heated to reflux overnight. After the reaction, the THF solution was drained, and toluene was added for extraction. Concentrate the toluene extract and recrystallize at -30°C to obtain a white solid that is the rare earth chloride Cp* 2 YCl 2 Li(THF) 2 .
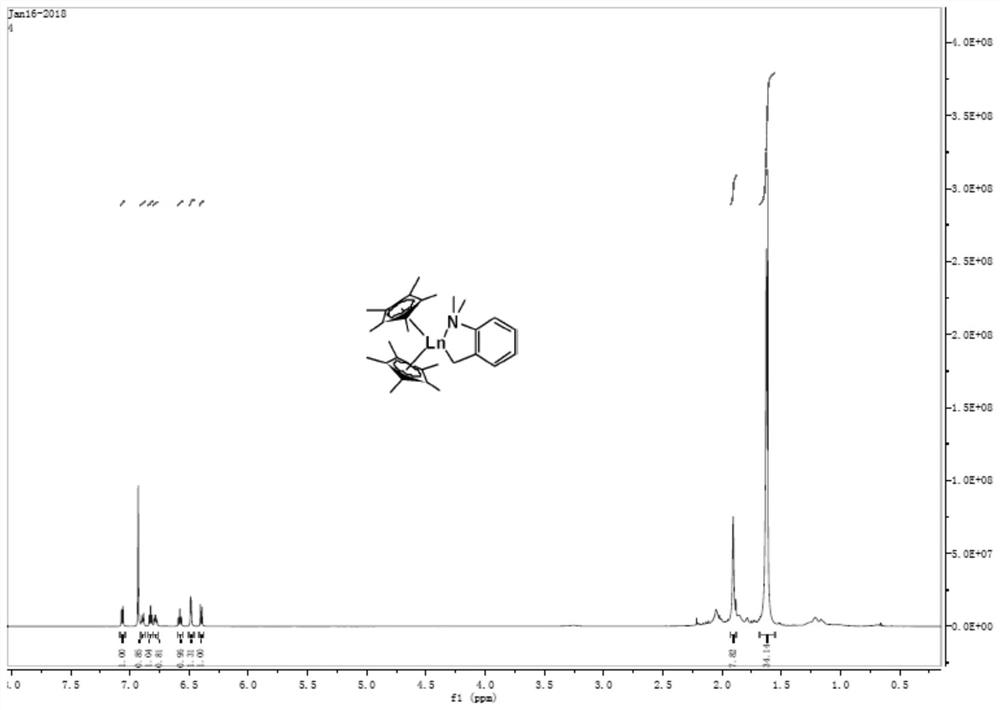
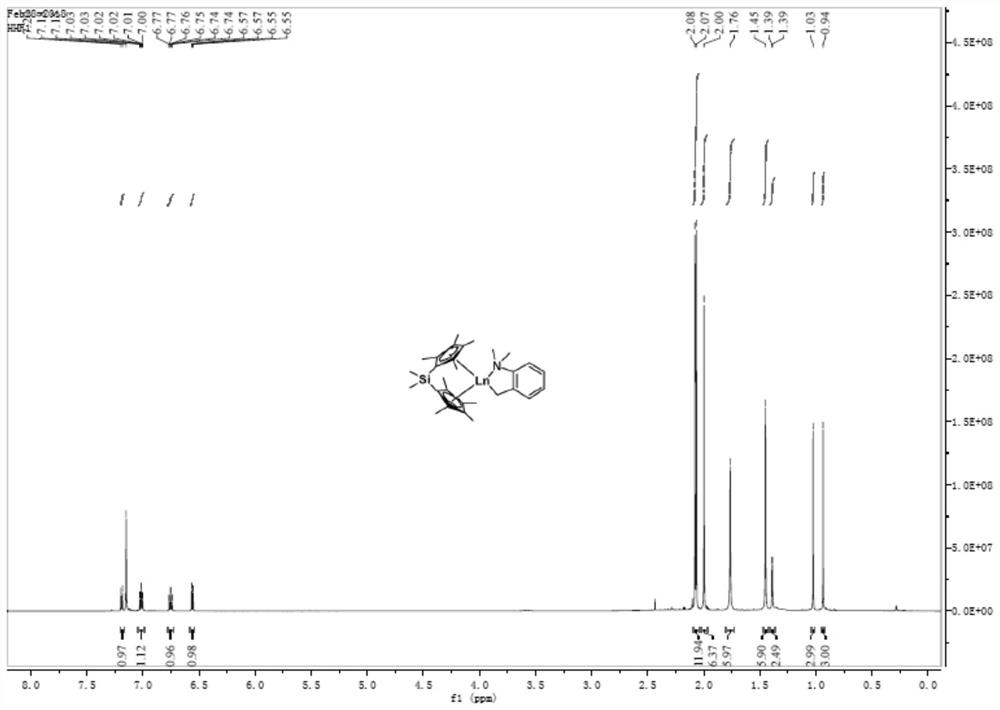
[0042] At -30°C, a toluene solution of o-dimethylaminobenzyllithium (AbzLi) was slowly added dropwise to Cp* 2 YCl 2 Li(T...
Embodiment 2
[0045] Preparation of mononuclear rare earth complex 2
[0046]
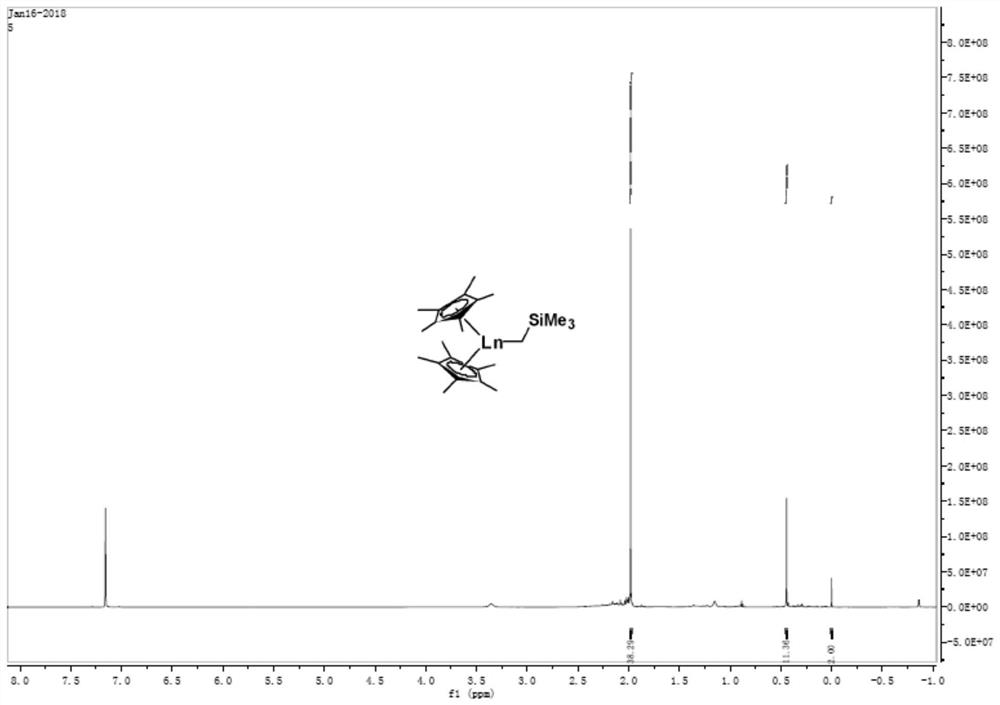
[0047] At -30°C, alkyllithium (TMSCH 2 The toluene solution of Li) is slowly added dropwise to Cp* 2 YCl 2 Li(THF) 2 In the toluene solution, the reaction was continued for 4 h after naturally rising to room temperature. After the reaction is completed, filter with a sand core funnel, and the filtrate is dried under vacuum to obtain a yellow oil, which is washed with a small amount of n-hexane to obtain a light yellow powder, which is Cp* 2 YACH 2 TMS product (compound 2).
[0048] NMR data: 1 H-NMR (600MHz, C6D6): 0.00 (s, 2H, CH2TMS), 0.44 (s, 9H, SiMe3), 1.98 (s, 30H, Cp-Me).
Embodiment 3
[0050] Preparation of mononuclear rare earth complex 3
[0051]
[0052] The synthesis of compound 3 refers to the synthesis of compound 1, YCl in the starting material 3 .nTHF replaced with GdCl 3 .nTHF, the experimental procedure is the same, the product obtained is light yellow powder solid. Due to the paramagnetic characteristics of the +3-valent Gd compound, NMR characterization cannot be performed, only for Cp* 2 GdCl 2 Li(THF) 2 X-ray single crystal diffraction characterization was carried out.
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com