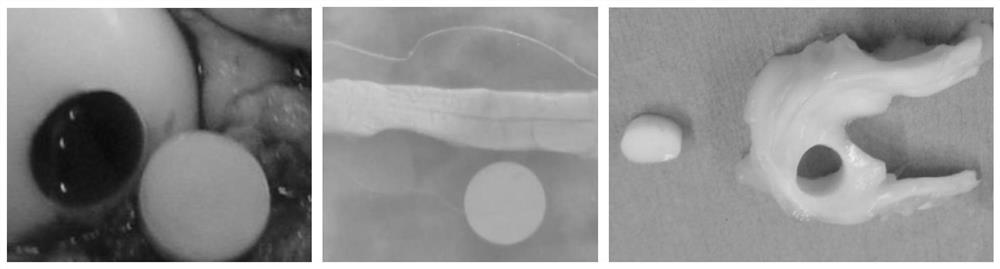
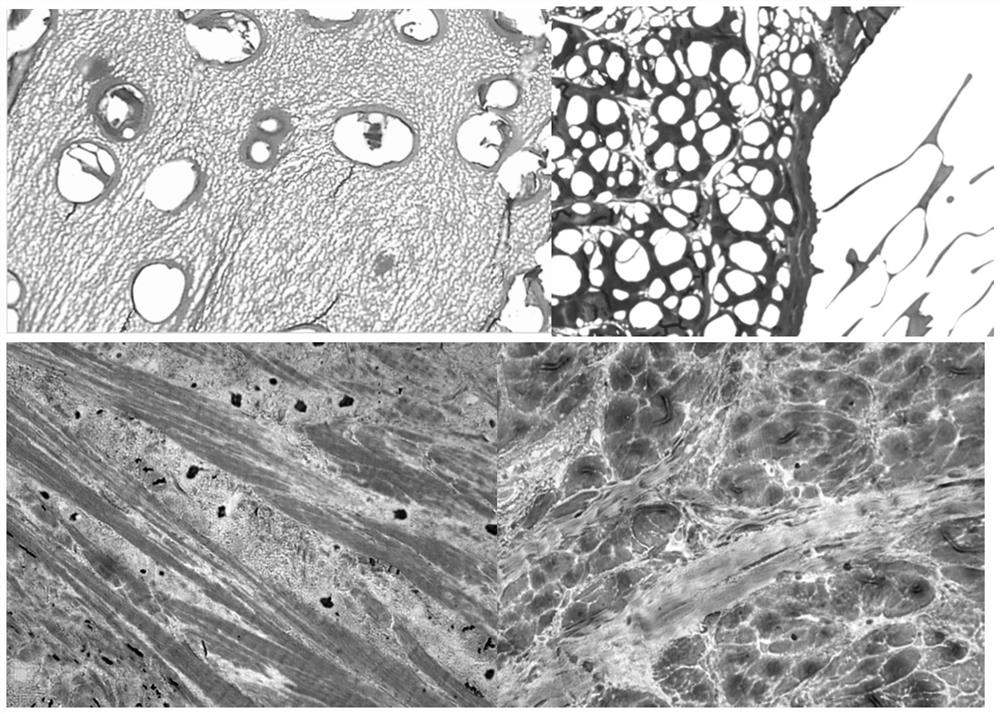
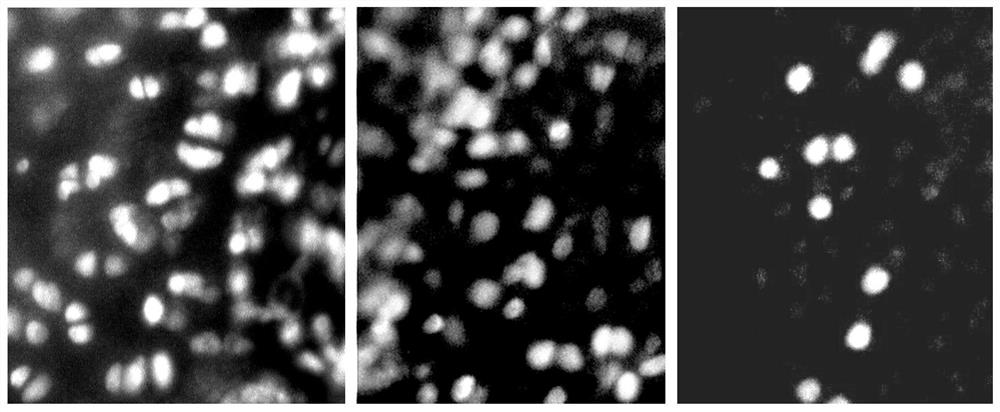
A kind of ice crystal-free cryopreservation solution and freezing method for cartilage, tendon and meniscus preservation
A technology of cryopreservation solution and meniscus, which is applied in the field of biomedical engineering, can solve the problems of being difficult to apply to the transportation environment in a long-term vibration state, low activity rate of articular cartilage cells, and short storage time, so as to improve the activity rate of cells and reduce Re-formation of ice crystals, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 1. Get 2.5mol ethylene glycol, 2.5mol propylene glycol, 0.5mol hydroxyethylpiperazine ethanesulfonic acid, 25 μmol Caspase inhibitor Z-VAD-FMK, 6mg matrix metalloproteinase inhibitor GM6001 and 0.8g polyvinyl alcohol, mix After uniformity, add Euro-Collin solution to make up to 1L to form preservation solution 1.
[0061] 2. Take an appropriate amount of preservation solution 1 from step 1 and cool it to 2-4°C. Add the cooled preservation solution 1 to the tissue to be frozen gradually in 6 steps at 2-4°C, and complete the introduction within 90 minutes (at 2-4°C, step by step in 15 minutes, step by step in 6 steps) Incrementally add preservation solution 1 to the tissue, 15 min each time).
[0062] 3. Cover the tissue that was introduced into Preservation Solution 1 in the previous step with 2-methylbutane. Place the 2-methylbutane-covered Preservative Solution 1 and the tissue container in the 2-methylbutane liquid bath and wait to freeze.
[0063]4. Introduce the ...
Embodiment 2
[0068] 1. Take 2.5mol ethylene glycol, 2.5mol propylene glycol, 0.5mol hydroxyethylpiperazine ethanesulfonic acid, 25μmol Caspase inhibitor Z-VAD-FMK, 6mg matrix metalloproteinase inhibitor GM6001, mix well and add Euro-Collin solution Dilute to 1L to form preservation solution 2.
[0069] 2. Take an appropriate amount of preservation solution 2 in step 1 and cool it to 2-4°C. Add the cooled preservation solution 2 gradually to the tissue to be frozen according to the 6-step method at 2-4°C, and complete the introduction within 90 minutes (at 2-4°C, step by step in 15 minutes, follow the 6-step method Incrementally add preservation solution 2 to the tissue, 15 min each time).
[0070] 3. Cool the tissue that was introduced into preservation solution 2 in the previous step to -100°C at a rate of -43°C / min, and then cool the sample to less than -130°C at a rate of -3±0.2°C / min, and then store the sample at 2 - in a liquid bath of methylbutane and kept at this temperature for 8...
Embodiment 3
[0075] 1. Take 2.5mol ethylene glycol, 2.5mol propylene glycol, 0.5mol hydroxyethylpiperazine ethanesulfonic acid, 6mg matrix metalloproteinase inhibitor GM6001 and 0.8g polyvinyl alcohol, mix well and add Euro-Collin solution to volume to 1L to form preservation solution 3.
[0076] Others are the same as embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com