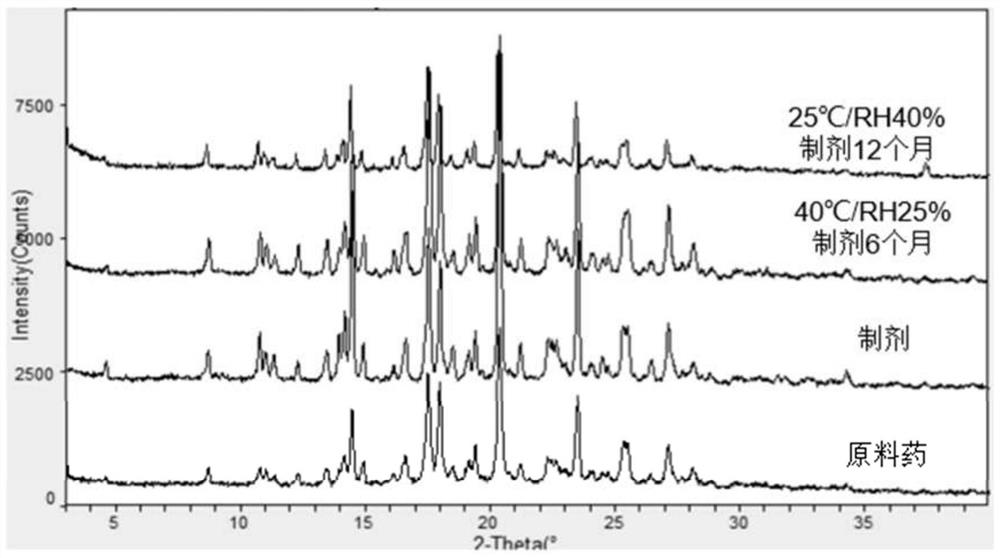
Suspension of triazole antifungal drug for atomizer
A technology of antifungal drugs and triazoles, which can be used in antifungal agents, liquid delivery, pharmaceutical formulations, etc., and can solve problems such as poor suspension stability, unsuitability for medicinal use, and drug particle agglomeration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 100kg itraconazole suspension for co-atomizer
[0049] (1) Add about 90kg of water for injection in the preparation tank, add 500g of Tween 80, stir to dissolve completely;
[0050] (2) Add 800g sodium chloride, 10g disodium edetate, 180g disodium hydrogen phosphate and 940g sodium dihydrogen phosphate, stir to dissolve completely;
[0051] (3) Add water to 99.5kg, stir to mix evenly, measure the pH value to 6.0, and obtain the auxiliary material solution;
[0052] (4) Pass the excipient solution through a 0.2 μm sterile filter, and then transfer it to a sterile storage tank;
[0053] (5) Put 500 g of sterile itraconazole bulk drug into the sterile-filtered excipient solution under the condition of A-level laminar flow protection; stir or homogenize to make itraconazole disperse evenly;
[0054] (6) Aseptic filling and sealing.
Embodiment 2
[0055] Example 2 100kg itraconazole suspension for co-atomizer
[0056] (1) Add about 90kg of water for injection in the preparation tank, add 100g of Tween 80, stir to dissolve completely;
[0057] (2) Add 800g sodium chloride, 10g disodium edetate, 180g disodium hydrogen phosphate and 940g sodium dihydrogen phosphate, stir to dissolve completely;
[0058] (3) Add water to 99.5kg, stir to mix evenly, measure the pH value to 6.0, and obtain the auxiliary material solution;
[0059] (4) Pass the excipient solution through a 0.2 μm sterile filter, and then transfer it to a sterile storage tank;
[0060] (5) Put 500 g of sterile itraconazole bulk drug into the excipient solution after sterilizing and filtering under the condition of A-level laminar flow protection. Stir or homogenize to disperse itraconazole evenly;
[0061] (6) Aseptic filling and sealing.
Embodiment 3
[0062] Example 3 100kg itraconazole suspension for co-atomizer
[0063] (1) Add about 90kg of water for injection in the preparation tank, add 25g of Tween 80, stir to dissolve completely;
[0064] (2) Add 800g sodium chloride, 10g disodium edetate, 180g disodium hydrogen phosphate and 940g sodium dihydrogen phosphate, stir to dissolve completely;
[0065] (3) Add water to 99.5kg, stir to mix evenly, measure the pH value to 6.0, and obtain the auxiliary material solution;
[0066] (4) Pass the excipient solution through a 0.2 μm sterile filter, and then transfer it to a sterile storage tank;
[0067] (5) Put 500 g of sterile itraconazole bulk drug into the excipient solution after sterilizing and filtering under the condition of A-level laminar flow protection. Stir or homogenize to disperse itraconazole evenly;
[0068] (6) Aseptic filling and sealing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com