Pharmaceutical composition, and preparation method and application thereof
A composition and drug technology, applied in the field of medicine, can solve problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Preparation of PLG-g-mPEG / DOX nanoparticles (referred to as DOX NPs)
[0075] The molecular weights of polyethylene glycol and polyglutamic acid were respectively selected to be 5000Da and 21600Da, and the drug carrier PLG-g-mPEG was dissolved in dimethyl sulfoxide solution at a concentration of 20 mg / mL. Dissolve commercially available doxorubicin hydrochloride DOX in dimethyl sulfoxide solution at a concentration of 10 mg / mL. Mix the carrier solution and the drug solution according to the corresponding mass ratio of 2:1, then slowly drop the mixed solution into ultrapure water, the volume of ultrapure water used is eight times the volume of dimethyl sulfoxide, and then stir for 20min, stir The mixed solution was placed in a 3500 molecular weight dialysis bag and dialyzed for 2 days to prepare PLG-g-mPEG / DOX nanoparticles. The particle size of the prepared nanoparticles is 100 nm. The drug loading capacity is 28.6%, and the drug loading rate is 80.3%. The ...
Embodiment 2
[0078] Example 2 Preparation of PEG / PLG / PEI / Spam1+shPD-L1 (Spam1+shPD-L1NPs for short)
[0079] The molecular weights of polyethyleneimine and polyglutamic acid were respectively selected as 25KDa and 2000Da, PEI was dissolved in the aqueous solution at a concentration of 5mg / mL, and polyglutamic acid was dissolved in the aqueous solution at a concentration of 1mg / mL. The two are mixed according to the mass ratio PEI:PLG=5:1. Vortex to mix well, compound for 15 minutes to form PEI / PLG nanoparticles. Mix the Spam1 plasmid and the shPD-L1 plasmid in equal amounts to form an aqueous solution, and the plasmid concentration is 1 mg / mL respectively. Mix the plasmid mixed solution with the PEG / PLG nanoparticle solution, vortex and mix well, and compound for 15 minutes. Dialdehyde PEG was dissolved in water at a concentration of 10 mg / mL. And add it to the previous plasmid nanoparticles, vortex and mix well, and compound for 15 minutes. The final mass ratio of each substance is: P...
Embodiment 3
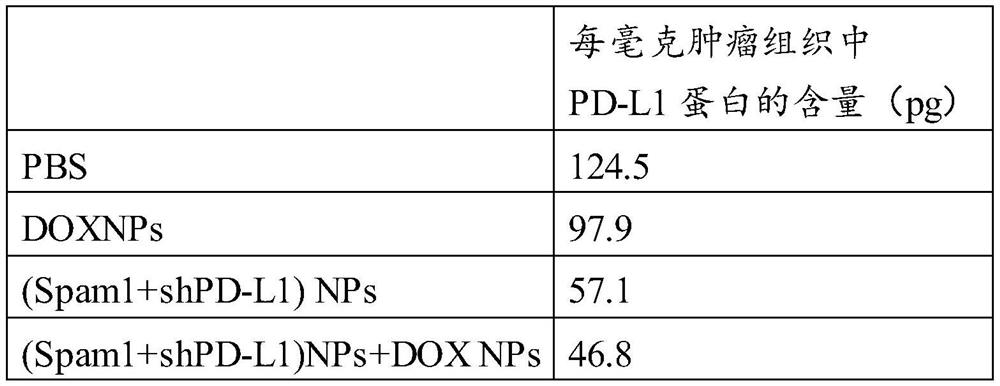
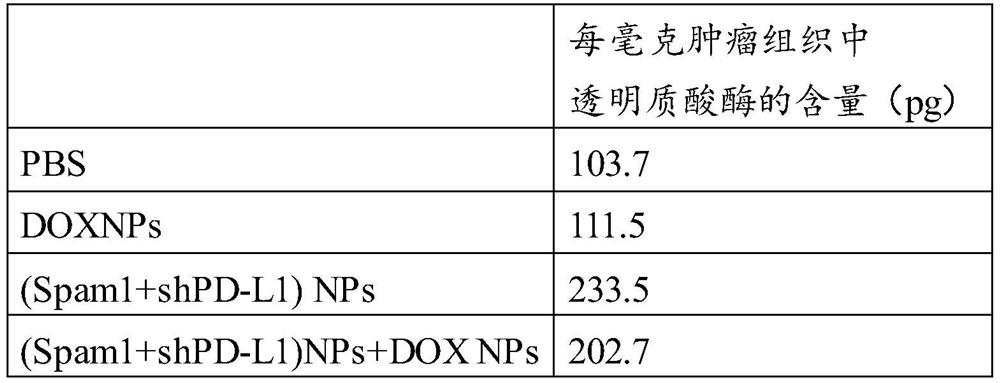
[0080] Example 3 Anti-tumor therapy of DOX NPs combined with Spam1+shPD-L1NPs
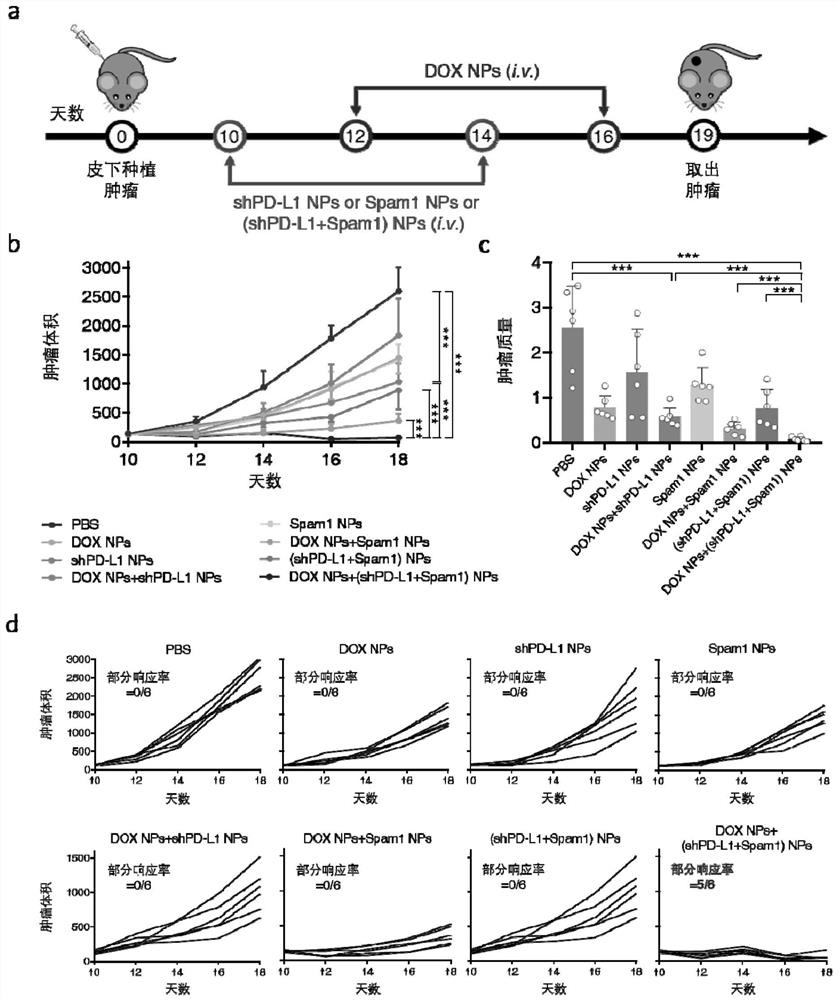
[0081] C57BL6 mice aged 4-6 weeks were randomly divided into 8 groups, 6 mice in each group. One million B16F10 cells were injected subcutaneously to construct a subcutaneous tumor model. Each group of mice received different treatments, and the experimental scheme was as follows: figure 1 As shown in a:
[0082] The mice in the PBS group were used as a control, and were only given the same amount of PBS buffer;
[0083] The dose of DOX NPs group was 5mg / kg;
[0084] The dose of shPD-L1NPs group was 0.75mg / kg;
[0085] DOX NPs+shPD-L1Nps group, according to figure 1 As shown in middle a, 5 mg / kg of DOX NPs and 0.75 mg / kg of shPD-L1NPs were administered;
[0086] The dosage of Spam1 NPs group was 0.75mg / kg;
[0087] DOX NPs+Spam1NPs group, according to figure 1 As shown in a, 5mg / kg of DOXNPs and 0.75mg / kg of Spam1NPs were given;
[0088] (shPD-L1+Spam1)NPs group, according to figure 1 As sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com