Diaphorase mutant with high thermal stability, diaphorase mutant gene with high thermal stability and preparation method of diaphorase mutant
A technology with high thermal stability and diaphorase, applied in the field of bioengineering, can solve the problems of high price, low output, inconvenient large-scale production, etc., achieve the effect of wide application range of pH and reduce production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 has the design of the diaphorase mutant gene of high thermostability
[0062] Based on the amino acid sequence of the diaphorase mutant with high thermostability of the present invention, the sequence is optimized according to the codon preference of Escherichia coli to obtain a mutant gene of diaphorase with high thermostability, which has high thermostability The amino acid sequence of the positive diaphorase mutant is shown in SEQ ID NO.1, and the nucleotide sequence of the optimized diaphorase mutant gene is shown in SEQ ID NO.2.
[0063] The sequence structure of said SEQ ID NO.1 is as follows:
[0064] MAKVLYITAHPHDDTQSYSMAVGKAFIETYKQVHPDHEVIHLDLYKEYIPEIDDVDVFSGWGKLRSGQSFEQLSAEEKTKVGRMNELCDQFISADKYVFVTPMWNFSFPPVLKAYIDAVAVADKTFKYTAQGPIGLLTDKKALHIQARGGFYSEGPAAQMEMGHELGRILEIIMFAARQFFGKVPSFEEETF.
[0065] The sequence structure of said SEQ ID NO.2 is as follows:
[0066] Atggctaaagttctgtacatcaccgctcacccgcacgacgacacccagtcttactctatggctgttggtaaagctttcatcga...
Embodiment 2
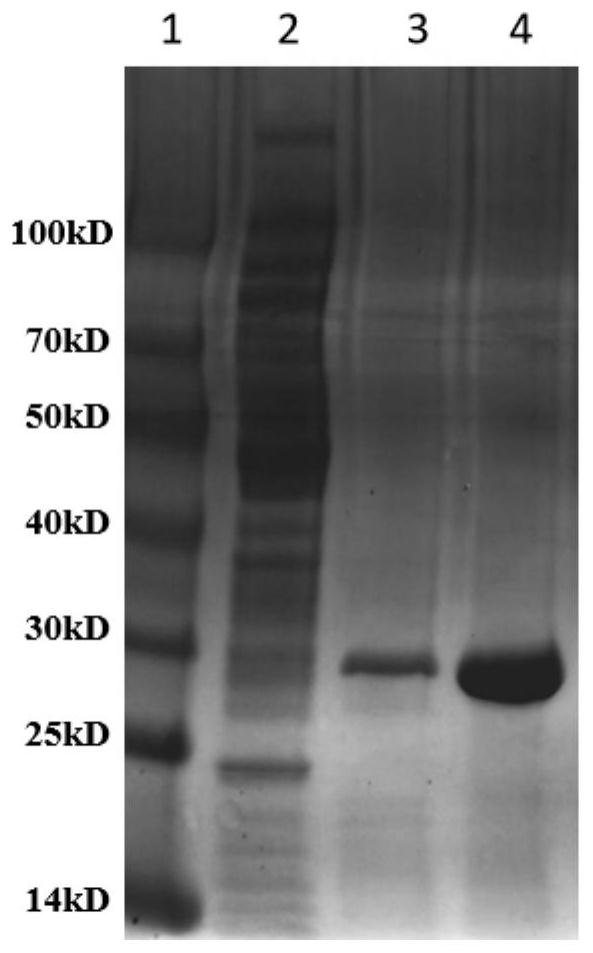
[0067] Embodiment 2 has the preparation of the diaphorase mutant of high thermostability
[0068] The preparation method of the diaphorase mutant with high thermostability described in this embodiment comprises the following steps:
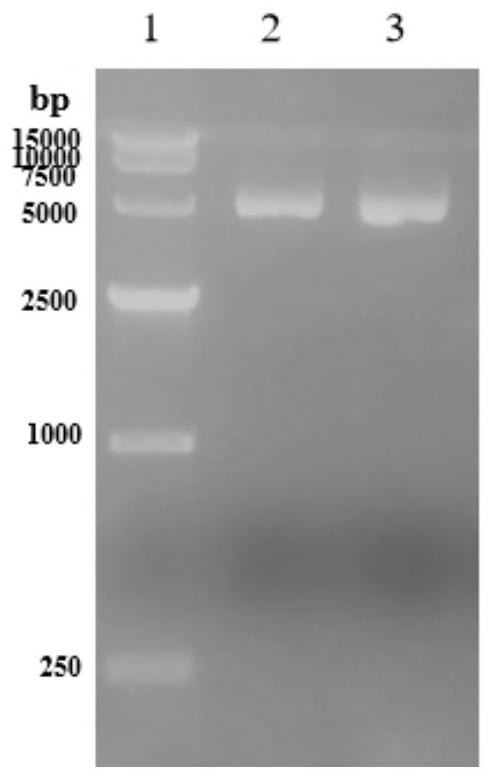
[0069] S1. Construction of high thermostability diaphorase mutant genetically engineered strains:
[0070] S101. Carrying out the whole gene synthesis of the diaphorase mutant gene according to the sequence of SEQ ID NO: 2, and amplifying the diaphorase mutant gene through the upstream primer and the downstream primer using the diaphorase mutant gene as a template, wherein, The upstream primer has a sequence as described in SEQ ID NO: 4, and the downstream primer has a sequence as described in SEQ ID NO: 5,
[0071] The sequence structure of said SEQ ID NO: 4 is as follows:
[0072] 5'-GGGAATTCCATATGATGGCTAAACTGCTG-3'.
[0073] The sequence structure of said SEQ ID NO: 5 is as follows:
[0074] 5'-CCGCTCGAGTTAGAAGTTTTTAGC-3'.
[0075] S102. A...
Embodiment 3
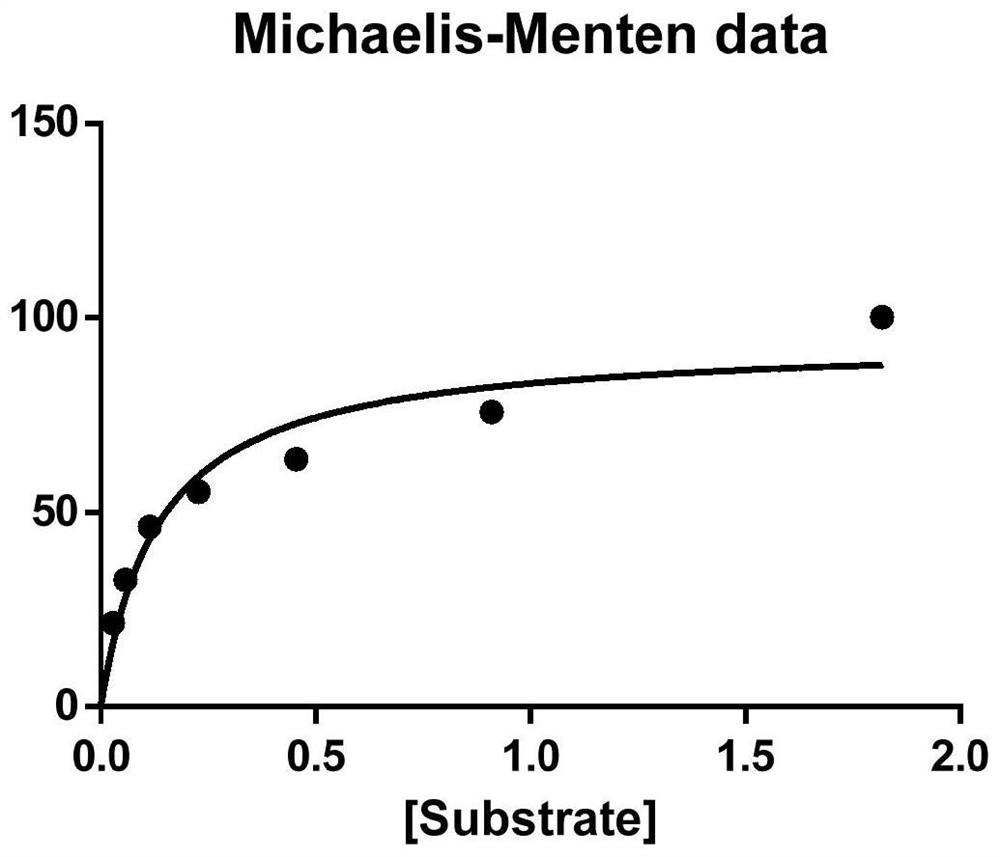
[0084] The enzymatic property determination of embodiment 3 high thermostability diaphorase mutants
[0085] Method for assaying activity of diaphorase mutants:
[0086] Step 1. Reaction mixture configuration: 0.50ml of 0.2M KH 2 PO 4 -NaOH solution (pH 7.5), 0.10ml of 0.25% (W / V) NTB (nitro blue tetrazolium) solution, 0.10ml of 1% (W / V) BSA solution, 0.10ml of 10mM NADH solution, 0.20ml distilled water. The reaction mixture should be prepared immediately and placed on ice.
[0087] Step 2. Determination of enzyme activity: Take 1ml of the above reaction mixture, put it in a 1.2ml cuvette, put it in a spectrophotometer at 37°C for 3min, add 100μL of diluted enzyme, react at 37°C for 7 minutes, and continuously measure the absorbance at 550nm . Record the absorbance change ΔA within 1 min.
[0088] Definition of diaphorase mutant enzyme activity: 1 enzyme activity unit is equal to the amount of enzyme required to oxidize 1 μM NADH in 1 min under the above reaction conditi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com